Deleted member 43589
Well-Known Member
I guess bodybuilders who eat huge amounts of protein have been right all along. So much for the 20g of protein bro science.
Jorn Trommelen, Glenn A.A. van Lieshout, Jean Nyakayiru, Andrew M. Holwerda, Joey S.J. Smeets, Floris K. Hendriks, Janneau M.X. van Kranenburg, Antoine H. Zorenc, Joan M. Senden, Joy P.B. Goessens, Annemie P. Gijsen, Luc J.C. van Loon, The anabolic response to protein ingestion during recovery from exercise has no upper limit in magnitude and duration in vivo in humans, Cell Reports Medicine, Volume 4, Issue 12, 2023,101324, ISSN 2666-3791, Redirecting.

 www.sciencedirect.com
www.sciencedirect.com
Summary
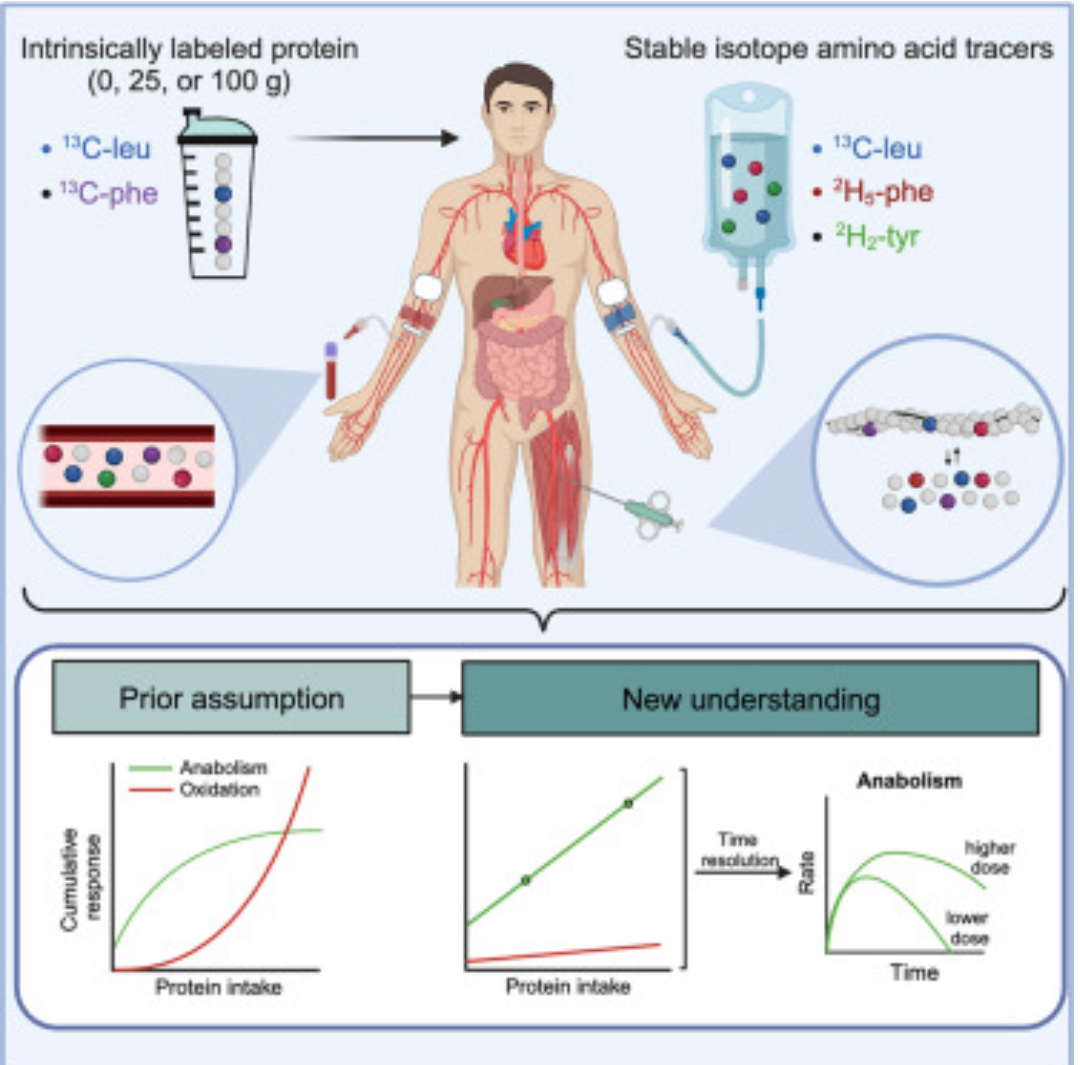
The belief that the anabolic response to feeding during postexercise recovery is transient and has an upper limit and that excess amino acids are being oxidized lacks scientific proof. Using a comprehensive quadruple isotope tracer feeding-infusion approach, we show that the ingestion of 100 g protein results in a greater and more prolonged (>12 h) anabolic response when compared to the ingestion of 25 g protein. We demonstrate a dose-response increase in dietary-protein-derived plasma amino acid availability and subsequent incorporation into muscle protein. Ingestion of a large bolus of protein further increases whole-body protein net balance, mixed-muscle, myofibrillar, muscle connective, and plasma protein synthesis rates. Protein ingestion has a negligible impact on whole-body protein breakdown rates or amino acid oxidation rates. These findings demonstrate that the magnitude and duration of the anabolic response to protein ingestion is not restricted and has previously been underestimated in vivo in humans.
Discussion
Here, we show that the anabolic response to protein ingestion has no apparent upper limit in magnitude and duration in vivo in humans. We demonstrate that protein ingestion results in a dose-dependent increase in dietary-protein-derived amino acid availability and a concomitant increase in muscle and whole-body protein synthesis rates (Figure 7). The postprandial increase in plasma amino acid availability has a negligible impact on whole-body protein breakdown or postprandial amino acid oxidation rates. The postprandial increase in muscle protein synthesis rates following ingestion of a large amount of protein (100 g) was sustained well beyond the transient anabolic and catabolic myocellular signaling response to feeding. These data provide valuable mechanistic insight into the ongoing controversy of the impact of different feeding strategies as a means to optimize muscle tissue anabolism and/or metabolic health.
It is generally believed that the ingestion of ∼20 g protein maximizes postprandial muscle protein synthesis rates at rest and during recovery from exercise in healthy, young adults. However, the clinical evidence for this is based on dose-response studies with a small range of protein intakes (≤45 g) and relative short assessment periods (≤6 h).18,19,20 Here, we comprehensively assessed the time course of postprandial protein handling by applying a quadruple isotope tracer feeding-infusion approach in vivo in humans. To investigate potential upper limits and/or sustained elevation of postprandial protein metabolism, we provided the proposed optimal amount (25 g) and the largest amount of protein that we consider feasible to consume in a single meal (100 g) and evaluated postprandial protein handling throughout a prolonged 12-h assessment period. We observed higher plasma, muscle, and whole-body protein synthesis rates following the ingestion of 100 g protein when compared to 25 g protein and placebo, respectively. The greater metabolic responses were present during the early postprandial phase (0–4 h) but were even more pronounced during the prolonged postprandial phase (4–12 h). These data support our hypothesis that even very large amounts of dietary protein are effectively utilized to support postprandial tissue anabolism but require a more prolonged period for complete protein digestion and amino acid absorption to become available for incorporation into tissues.
It has been proposed that when dietary protein is consumed beyond the rate by which it can be utilized for protein synthesis, the excess amino acids will be directed toward oxidation.13,32 Our data do not provide any evidence for an upper limit to the whole-body protein synthetic response and, therefore, any disproportional increase in amino acid oxidation following the ingestion of a large amount of protein. In fact, postprandial amino acid oxidation rates were negligible when expressed relative to the increase in whole-body protein synthesis rates (Figure 2J). Therefore, we reassessed amino acid oxidation rates reported in previous dose-response studies by expressing them relative to protein intake.18,19 This revealed that postprandial amino acid oxidation rates represent a relative minor metabolic fate (<15% of the increment in ingested protein), supporting our findings that the majority of the ingested protein (>85%) is utilized for tissue protein synthesis. Furthermore, these findings seem robust, as they have been observed following ingestion of different types of protein while using different assessment techniques and amino acid tracers to assess amino acid oxidation rates (phenylalanine hydroxylation in the present study and by Witard et al.19 and breath 13CO2 production from leucine by Moore et al.18). Collectively, these data refute the notion that amino acid oxidation functions as a sink for excess amino acid provision.
In the present study, milk protein was applied because it is the food item with the largest contribution to daily protein intake in the Western world.42 Because milk protein consists of 20% rapid digestible whey protein and 80% slowly digestible casein protein, it could be speculated that a more prolonged anabolic response to protein ingestion is unique to slowly digestible proteins. More rapidly digestible proteins may be disproportionally oxidized and, as such, may result in less amino acids being available for de novo protein synthesis.3 However, previous dose-response studies that administered rapidly digestible protein observed that postprandial amino acid oxidation is actually very limited.18,19 Moreover, we observed no differences in muscle protein synthesis rates following the ingestion of relatively large doses of whey and casein protein,43 implying that more rapidly digestible proteins do not result in disproportional (high) amino acid oxidation rates. Collectively, these data indicate that the metabolic responses observed in the present study are unlikely to be restricted to more slowly digestible proteins and are generalizable to other proteins despite different digestion kinetics.
It has been well established that leucine is a key regulator of the mTOR1 pathway in various tissues.17 We observed that the ingestion of a large amount of protein resulted in an increase in plasma amino acid availability (including leucine). This increase in plasma amino acid availability was not mirrored by an increase in free amino acid concentrations in muscle tissue, as only muscle free branched-chain amino acids concentrations (leucine, isoleucine, and valine) were elevated. Despite the sustained intra- and extracellular increase in leucine availability, the postprandial increase in anabolic myocellular signaling was transient. These data support prior evidence that increased leucine availability plays a critical role in the initial (<4 h) stimulation of postprandial tissue anabolism. Importantly, we extend on this work by showing that prolonged leucine availability does not result in prolonged mTOR activation and that enhanced anabolic signaling is not required to perpetuate the postprandial increase in muscle protein synthesis rate.
We observed that the plasma, muscle, and whole-body protein synthetic responses increased in relation to the magnitude and timeline of the postprandial amino acid response to feeding. This implies that following the activation of anabolic signaling by the postprandial increase in leucine availability, protein synthesis rates are mainly modulated by the availability of amino acids as substrate. In line, we observed that the anabolic responses to feeding corresponded to postprandial plasma amino kinetics (i.e., rate of plasma amino acid appearance and disappearance). Furthermore, we demonstrate for the first time that protein ingestion has a minor impact on endogenous amino acid fluxes, with exogenous amino acid fluxes responding in line with the amount of protein ingested. The relative contribution of exogenous-protein-derived amino acids to overall amino acid release into the circulation, plasma amino acid uptake into tissues, and amino acid incorporation into myofibrillar protein were remarkably similar (32%, 30%, and 27%, respectively, following ingestion of 100 g protein). Collectively, these data demonstrate that protein ingestion results in a coordinated flux of exogenous-protein-derived amino acids toward incorporation in tissue protein that is additive to the endogenous amino acid flux.
Meal frequency has been proposed as an important modulator of tissue and whole-body metabolism. For example, dietary guidelines in both health and disease typically recommend an equal distribution of daily protein requirements over the main meals to support muscle anabolism.21,22 These recommendations are based entirely on the belief that the muscle protein synthetic response to ingestion of a single bolus of protein has a ceiling and is short lived. The current findings provide evidence to support more flexibility in feeding patterns aimed at enhancing muscle anabolism. Specifically, we show that the ingestion of a single large amount of protein is followed by a prolonged anabolic response, which would obviate the need to consume another protein-rich meal in close temporal proximity. This may explain why time-restricted feeding patterns do not seem to compromise muscle mass maintenance.44,45,46 Our data suggest that time-restricted feeding may not result in a postabsorptive state until far beyond the end of the feeding window. This is of relevance since time-restricted feeding is typically applied to avoid prolonged postprandial periods, which are believed to be undesirable for metabolic health.47,48 Furthermore, it has been speculated that sustained anabolism and mTOR activation inhibit clearance of compromised proteins.49 However, we observed that the ingestion of a single, large amount of protein (100 g) resulted in prolonged anabolism without compromising whole-body protein breakdown rates, muscle mTOR signaling, or markers of muscle autophagy. Collectively, our data suggest that time-restricted feeding protocols likely overestimate the length of their postabsorptive windows but also that a postabsorptive state is not required to allow protein clearance.
Jorn Trommelen, Glenn A.A. van Lieshout, Jean Nyakayiru, Andrew M. Holwerda, Joey S.J. Smeets, Floris K. Hendriks, Janneau M.X. van Kranenburg, Antoine H. Zorenc, Joan M. Senden, Joy P.B. Goessens, Annemie P. Gijsen, Luc J.C. van Loon, The anabolic response to protein ingestion during recovery from exercise has no upper limit in magnitude and duration in vivo in humans, Cell Reports Medicine, Volume 4, Issue 12, 2023,101324, ISSN 2666-3791, Redirecting.

The anabolic response to protein ingestion during recovery from exercise has no upper limit in magnitude and duration in vivo in humans
The belief that the anabolic response to feeding during postexercise recovery is transient and has an upper limit and that excess amino acids are bein…
 www.sciencedirect.com
www.sciencedirect.com
Summary
The belief that the anabolic response to feeding during postexercise recovery is transient and has an upper limit and that excess amino acids are being oxidized lacks scientific proof. Using a comprehensive quadruple isotope tracer feeding-infusion approach, we show that the ingestion of 100 g protein results in a greater and more prolonged (>12 h) anabolic response when compared to the ingestion of 25 g protein. We demonstrate a dose-response increase in dietary-protein-derived plasma amino acid availability and subsequent incorporation into muscle protein. Ingestion of a large bolus of protein further increases whole-body protein net balance, mixed-muscle, myofibrillar, muscle connective, and plasma protein synthesis rates. Protein ingestion has a negligible impact on whole-body protein breakdown rates or amino acid oxidation rates. These findings demonstrate that the magnitude and duration of the anabolic response to protein ingestion is not restricted and has previously been underestimated in vivo in humans.
Discussion
Here, we show that the anabolic response to protein ingestion has no apparent upper limit in magnitude and duration in vivo in humans. We demonstrate that protein ingestion results in a dose-dependent increase in dietary-protein-derived amino acid availability and a concomitant increase in muscle and whole-body protein synthesis rates (Figure 7). The postprandial increase in plasma amino acid availability has a negligible impact on whole-body protein breakdown or postprandial amino acid oxidation rates. The postprandial increase in muscle protein synthesis rates following ingestion of a large amount of protein (100 g) was sustained well beyond the transient anabolic and catabolic myocellular signaling response to feeding. These data provide valuable mechanistic insight into the ongoing controversy of the impact of different feeding strategies as a means to optimize muscle tissue anabolism and/or metabolic health.
It is generally believed that the ingestion of ∼20 g protein maximizes postprandial muscle protein synthesis rates at rest and during recovery from exercise in healthy, young adults. However, the clinical evidence for this is based on dose-response studies with a small range of protein intakes (≤45 g) and relative short assessment periods (≤6 h).18,19,20 Here, we comprehensively assessed the time course of postprandial protein handling by applying a quadruple isotope tracer feeding-infusion approach in vivo in humans. To investigate potential upper limits and/or sustained elevation of postprandial protein metabolism, we provided the proposed optimal amount (25 g) and the largest amount of protein that we consider feasible to consume in a single meal (100 g) and evaluated postprandial protein handling throughout a prolonged 12-h assessment period. We observed higher plasma, muscle, and whole-body protein synthesis rates following the ingestion of 100 g protein when compared to 25 g protein and placebo, respectively. The greater metabolic responses were present during the early postprandial phase (0–4 h) but were even more pronounced during the prolonged postprandial phase (4–12 h). These data support our hypothesis that even very large amounts of dietary protein are effectively utilized to support postprandial tissue anabolism but require a more prolonged period for complete protein digestion and amino acid absorption to become available for incorporation into tissues.
It has been proposed that when dietary protein is consumed beyond the rate by which it can be utilized for protein synthesis, the excess amino acids will be directed toward oxidation.13,32 Our data do not provide any evidence for an upper limit to the whole-body protein synthetic response and, therefore, any disproportional increase in amino acid oxidation following the ingestion of a large amount of protein. In fact, postprandial amino acid oxidation rates were negligible when expressed relative to the increase in whole-body protein synthesis rates (Figure 2J). Therefore, we reassessed amino acid oxidation rates reported in previous dose-response studies by expressing them relative to protein intake.18,19 This revealed that postprandial amino acid oxidation rates represent a relative minor metabolic fate (<15% of the increment in ingested protein), supporting our findings that the majority of the ingested protein (>85%) is utilized for tissue protein synthesis. Furthermore, these findings seem robust, as they have been observed following ingestion of different types of protein while using different assessment techniques and amino acid tracers to assess amino acid oxidation rates (phenylalanine hydroxylation in the present study and by Witard et al.19 and breath 13CO2 production from leucine by Moore et al.18). Collectively, these data refute the notion that amino acid oxidation functions as a sink for excess amino acid provision.
In the present study, milk protein was applied because it is the food item with the largest contribution to daily protein intake in the Western world.42 Because milk protein consists of 20% rapid digestible whey protein and 80% slowly digestible casein protein, it could be speculated that a more prolonged anabolic response to protein ingestion is unique to slowly digestible proteins. More rapidly digestible proteins may be disproportionally oxidized and, as such, may result in less amino acids being available for de novo protein synthesis.3 However, previous dose-response studies that administered rapidly digestible protein observed that postprandial amino acid oxidation is actually very limited.18,19 Moreover, we observed no differences in muscle protein synthesis rates following the ingestion of relatively large doses of whey and casein protein,43 implying that more rapidly digestible proteins do not result in disproportional (high) amino acid oxidation rates. Collectively, these data indicate that the metabolic responses observed in the present study are unlikely to be restricted to more slowly digestible proteins and are generalizable to other proteins despite different digestion kinetics.
It has been well established that leucine is a key regulator of the mTOR1 pathway in various tissues.17 We observed that the ingestion of a large amount of protein resulted in an increase in plasma amino acid availability (including leucine). This increase in plasma amino acid availability was not mirrored by an increase in free amino acid concentrations in muscle tissue, as only muscle free branched-chain amino acids concentrations (leucine, isoleucine, and valine) were elevated. Despite the sustained intra- and extracellular increase in leucine availability, the postprandial increase in anabolic myocellular signaling was transient. These data support prior evidence that increased leucine availability plays a critical role in the initial (<4 h) stimulation of postprandial tissue anabolism. Importantly, we extend on this work by showing that prolonged leucine availability does not result in prolonged mTOR activation and that enhanced anabolic signaling is not required to perpetuate the postprandial increase in muscle protein synthesis rate.
We observed that the plasma, muscle, and whole-body protein synthetic responses increased in relation to the magnitude and timeline of the postprandial amino acid response to feeding. This implies that following the activation of anabolic signaling by the postprandial increase in leucine availability, protein synthesis rates are mainly modulated by the availability of amino acids as substrate. In line, we observed that the anabolic responses to feeding corresponded to postprandial plasma amino kinetics (i.e., rate of plasma amino acid appearance and disappearance). Furthermore, we demonstrate for the first time that protein ingestion has a minor impact on endogenous amino acid fluxes, with exogenous amino acid fluxes responding in line with the amount of protein ingested. The relative contribution of exogenous-protein-derived amino acids to overall amino acid release into the circulation, plasma amino acid uptake into tissues, and amino acid incorporation into myofibrillar protein were remarkably similar (32%, 30%, and 27%, respectively, following ingestion of 100 g protein). Collectively, these data demonstrate that protein ingestion results in a coordinated flux of exogenous-protein-derived amino acids toward incorporation in tissue protein that is additive to the endogenous amino acid flux.
Meal frequency has been proposed as an important modulator of tissue and whole-body metabolism. For example, dietary guidelines in both health and disease typically recommend an equal distribution of daily protein requirements over the main meals to support muscle anabolism.21,22 These recommendations are based entirely on the belief that the muscle protein synthetic response to ingestion of a single bolus of protein has a ceiling and is short lived. The current findings provide evidence to support more flexibility in feeding patterns aimed at enhancing muscle anabolism. Specifically, we show that the ingestion of a single large amount of protein is followed by a prolonged anabolic response, which would obviate the need to consume another protein-rich meal in close temporal proximity. This may explain why time-restricted feeding patterns do not seem to compromise muscle mass maintenance.44,45,46 Our data suggest that time-restricted feeding may not result in a postabsorptive state until far beyond the end of the feeding window. This is of relevance since time-restricted feeding is typically applied to avoid prolonged postprandial periods, which are believed to be undesirable for metabolic health.47,48 Furthermore, it has been speculated that sustained anabolism and mTOR activation inhibit clearance of compromised proteins.49 However, we observed that the ingestion of a single, large amount of protein (100 g) resulted in prolonged anabolism without compromising whole-body protein breakdown rates, muscle mTOR signaling, or markers of muscle autophagy. Collectively, our data suggest that time-restricted feeding protocols likely overestimate the length of their postabsorptive windows but also that a postabsorptive state is not required to allow protein clearance.
Last edited:

















