madman
Super Moderator
ABSTRACT
Introduction: Hypogonadism is an important issue among the male population. Treatments such as exogenous testosterone have become very popular. One of the adverse effects of testosterone is its suppression of fertility. This has led to the use of alternative therapies such as selective estrogen receptor modulators (SERMs) that aim to correct hypogonadism without reducing fertility.
Areas covered: The SERM, clomiphene citrate, which is approved by the FDA for the treatment of ovarian dysfunction, has been shown to have beneficial effects on male hypogonadism. Clomiphene citrate exists as a mixture of both the cis-isomer (zuclomiphene) and the trans-isomer (enclomiphene). The literature has suggested that most of the beneficial effects of clomiphene are due to the trans-isomer enclomiphene. Zuclomiphene contributes little to the intended outcomes. The purpose of this drug profile is to examine the available literature on trans-isomer enclomiphene.
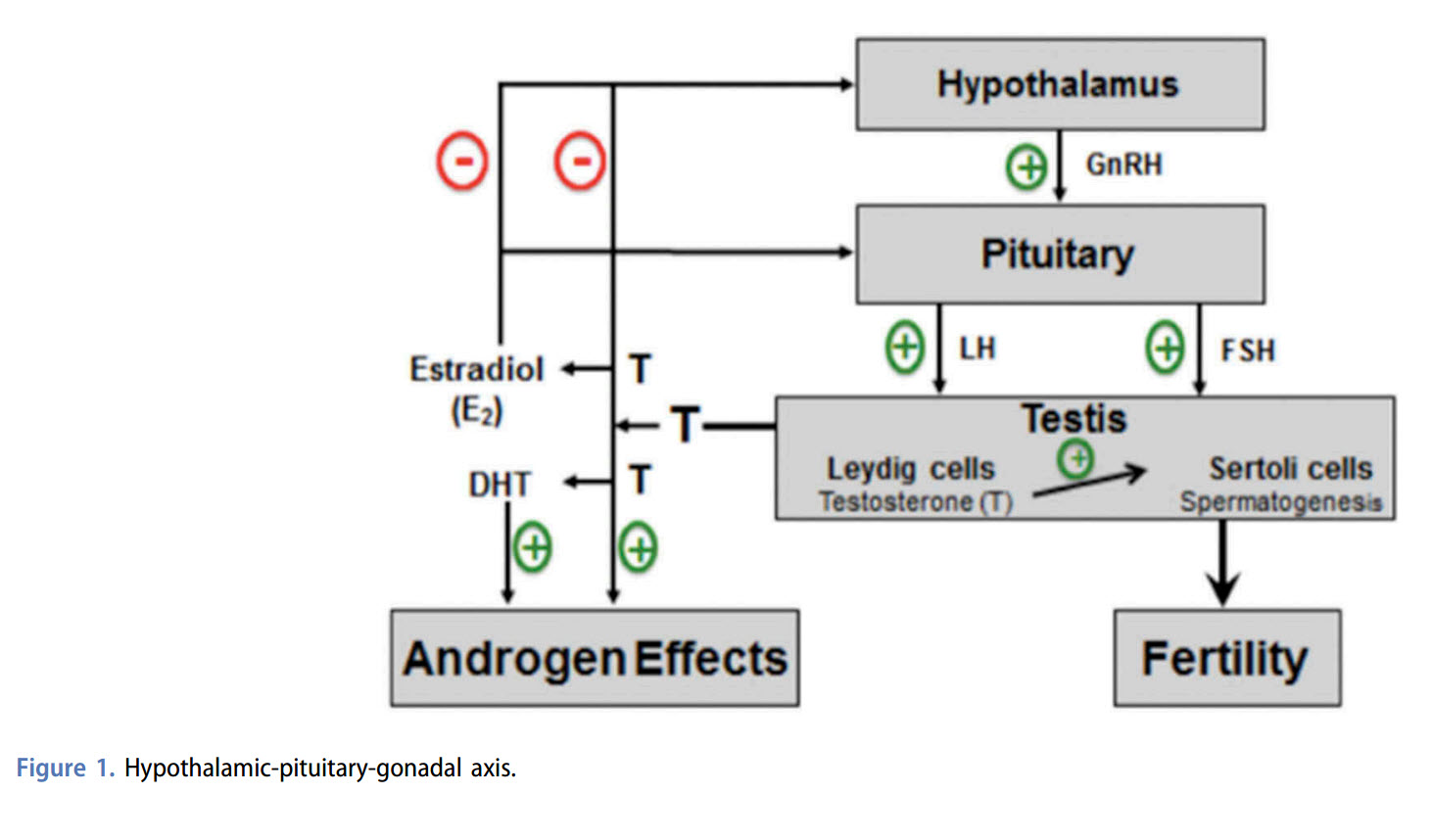
Expert opinion: Enclomiphene has been shown to increase testosterone levels while stimulating FSH and LH production. Initial studies demonstrated that enclomiphene maintains the androgenic benefit of clomiphene citrate without the undesirable effects attributable to zuclomiphene. This article reviews the difficulties associated with the FDA approval of a new molecular entity related to the treatment of hypogonadism.
Article highlights
● Clomiphene Citrate is used off-label to treat secondary hypogonadism in men desiring to preserve fertility
● The active isomer of clomiphene is enclomiphene while the other isomer zuclomiphene may actually antagonize the desired effects
● Enclomiphene has been shown to raise testosterone levels in a similar fashion to transdermal testosterone
● Enclomiphene has also been shown to preserve sperm concentration when compared with exogenous testosterone replacement
● No rigorous studies have demonstrated symptomatic benefits with enclomiphene
● The FDA denied the approval of enclomiphene on the grounds that no symptomatic benefit was identified
● Enclomiphene serves as an example of how difficult it is to gain approval for a new molecular entity for the treatment of secondary hypogonadism
In summary, enclomiphene is a very promising drug for patients with secondary hypogonadism and who are concerned about the negative effects of exogenous testosterone. Despite the fact that there was clearly demonstrated efficacy without any substantial safety issues. The lack of clearly demonstrated symptomatic improvement in phase III studies prevented enclomiphene from obtaining FDA approval. The difficulty of demonstrating a symptomatic benefit for secondary hypogonadism may prove a substantial barrier for years to come.
Introduction: Hypogonadism is an important issue among the male population. Treatments such as exogenous testosterone have become very popular. One of the adverse effects of testosterone is its suppression of fertility. This has led to the use of alternative therapies such as selective estrogen receptor modulators (SERMs) that aim to correct hypogonadism without reducing fertility.
Areas covered: The SERM, clomiphene citrate, which is approved by the FDA for the treatment of ovarian dysfunction, has been shown to have beneficial effects on male hypogonadism. Clomiphene citrate exists as a mixture of both the cis-isomer (zuclomiphene) and the trans-isomer (enclomiphene). The literature has suggested that most of the beneficial effects of clomiphene are due to the trans-isomer enclomiphene. Zuclomiphene contributes little to the intended outcomes. The purpose of this drug profile is to examine the available literature on trans-isomer enclomiphene.
Expert opinion: Enclomiphene has been shown to increase testosterone levels while stimulating FSH and LH production. Initial studies demonstrated that enclomiphene maintains the androgenic benefit of clomiphene citrate without the undesirable effects attributable to zuclomiphene. This article reviews the difficulties associated with the FDA approval of a new molecular entity related to the treatment of hypogonadism.
Article highlights
● Clomiphene Citrate is used off-label to treat secondary hypogonadism in men desiring to preserve fertility
● The active isomer of clomiphene is enclomiphene while the other isomer zuclomiphene may actually antagonize the desired effects
● Enclomiphene has been shown to raise testosterone levels in a similar fashion to transdermal testosterone
● Enclomiphene has also been shown to preserve sperm concentration when compared with exogenous testosterone replacement
● No rigorous studies have demonstrated symptomatic benefits with enclomiphene
● The FDA denied the approval of enclomiphene on the grounds that no symptomatic benefit was identified
● Enclomiphene serves as an example of how difficult it is to gain approval for a new molecular entity for the treatment of secondary hypogonadism
In summary, enclomiphene is a very promising drug for patients with secondary hypogonadism and who are concerned about the negative effects of exogenous testosterone. Despite the fact that there was clearly demonstrated efficacy without any substantial safety issues. The lack of clearly demonstrated symptomatic improvement in phase III studies prevented enclomiphene from obtaining FDA approval. The difficulty of demonstrating a symptomatic benefit for secondary hypogonadism may prove a substantial barrier for years to come.
Attachments
-
[email protected]1.2 MB · Views: 732

















