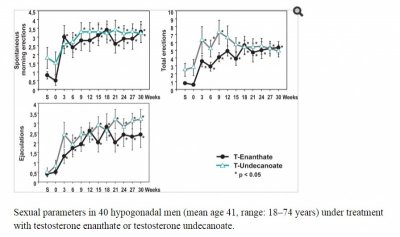
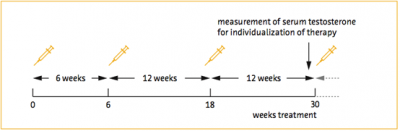
Nelson Vergel
Founder, ExcelMale.com
Update on Aveed (Nebido- testosterone undecanoate- long acting testosterone):
After a few problems with the FDA review process that have slowed down its approval by 3 years, this was the last announcement:
"Endo Pharmaceuticals Inc., a subsidiary of Endo Health Solutions Inc. (Nasdaq: ENDP) announced today that the U.S. Food and Drug Administration (FDA) has accepted for review the complete response submission made by Endo to the new drug application (NDA) for its long-acting testosterone undecanoate injection, Aveed, intended for men diagnosed with hypogonadism. In connection with the acceptance, the FDA assigned Endo's NDA a new Prescription Drug User Fee Act (PDUFA) action date of Feb. 28, 2014."
(NDA) for its long-acting testosterone undecanoate injection, Aveed, intended for men diagnosed with hypogonadism. In connection with the acceptance, the FDA assigned Endo's NDA a new Prescription Drug User Fee Act (PDUFA) action date of Feb. 28, 2014."
The following map shows the countries were it is approved (in orange)
After a few problems with the FDA review process that have slowed down its approval by 3 years, this was the last announcement:
"Endo Pharmaceuticals Inc., a subsidiary of Endo Health Solutions Inc. (Nasdaq: ENDP) announced today that the U.S. Food and Drug Administration (FDA) has accepted for review the complete response submission made by Endo to the new drug application
The following map shows the countries were it is approved (in orange)
Attachments
Last edited: