@smokedsalmon lol, sorry I had to laugh! Only because we just found out we’re pregnant. I had a Semen analysis done and my count was at 8 million and that’s only with 300IU’s 2x week which is JUST enough for me to maintain testicular size. Normal sperm count is 15-20 million so I thought we’re ok, nope, just found out we’re pregnant. Only takes one, LOL!
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Trying HCG once more - dosage and advice?
- Thread starter smokedsalmon
- Start date
-
- Tags
- hcg
@smokedsalmon my Endocrinologist did not have any scientific evidence to support this and she was upfront about it, this is purely anecdotal. Her advice was to make your HCG dose extremely concentrated. She said for some reason her male patients react better at more concentrated doses.
my dilution:
12000iu / 4ml Water = 3,000 iu per ml
300 per .1
I take .1 2x week.
This is the bare min I need to keep the boys alive.
I only bring this up because you said HCG didn’t work well for you before, so maybe changing up the dilution ratio would help. Again, no science behind this, just anecdotal. For me, a less concentrated does resulted in more flushed face/blushing in the face, some hot flashes. I tried the more concentrated does and that went away.
Just a thought!
my dilution:
12000iu / 4ml Water = 3,000 iu per ml
300 per .1
I take .1 2x week.
This is the bare min I need to keep the boys alive.
I only bring this up because you said HCG didn’t work well for you before, so maybe changing up the dilution ratio would help. Again, no science behind this, just anecdotal. For me, a less concentrated does resulted in more flushed face/blushing in the face, some hot flashes. I tried the more concentrated does and that went away.
Just a thought!
Your HCT is low given the amount of T you take and where your T levels are. What is your donation protocol (how often, whole blood, double reds, platelets)? TxI've always gotten the best symptom relief over 30 FT I've noticed. But when I start pushing it past 40, I notice I get really moody. My bloodwork still comes back good though. I do donate blood regularly too to keep this in check.
It's tough for me to titrate to symptoms with HCG as I really don't have any - just trying to preserve my balls for future use. No major shrinkage or anything. I do get some pain during orgasm sometimes, but that's rare due to an antidepressant I'm on (Nardil).
One more question - does dropping my 150mg week T dose to 100 and trying 125IU HCG every other day sound reasonable? Almost makes me wonder if my HCG side effects were from too much TT.
smokedsalmon
Member
Your HCT is low given the amount of T you take and where your T levels are. What is your donation protocol (how often, whole blood, double reds, platelets)? Tx
I donate every couple months - as much as they'll let me. Whole blood. Going today actually!
I'm waiting to get another TT/FT test back now to see if the last one was accurate. I've never seen my E2 so low for such a high TT so I'm questioning it.
madman
Super Moderator
I donate every couple months - as much as they'll let me. Whole blood. Going today actually!
I'm waiting to get another TT/FT test back now to see if the last one was accurate. I've never seen my E2 so low for such a high TT so I'm questioning it.
You need to take a step back and think about this!
Your ferritin is most likely crashed and can have a negative impact on thyroid function let alone lead to anemia.
If you are constantly trying to manage elevated RBCs/hemoglobin/hematocrit with frequent blood donations than you may need to cut back on your T dose slightly and bring your levels down some if they are truly as high as what you posted earlier.
Donating more than 2-3 times/year is a surefire way to tank your ferritin/iron.
You should have mentioned this in your first post.
Running a protocol that results in a very high TT/FT you are bound to run into elevated RBCs/hemoglobin/hematocrit let alone chasing your tail donating frequently to try and manage it.
smokedsalmon
Member
You need to take a step back and think about this!
Your ferritin is most likely crashed and can have a negative impact on thyroid function let alone lead to anemia.
If you are constantly trying to manage elevated RBCs/hemoglobin/hematocrit with frequent blood donations than you may need to cut back on your T dose slightly and bring your levels down some if they are truly as high as what you posted earlier.
Donating more than 2-3 times/year is a surefire way to tank your ferritin/iron.
You should have mentioned this in your first post.
Running a protocol that results in a very high TT/FT you are bound to run into elevated RBCs/hemoglobin/hematocrit let alone chasing your tail donating frequently to try and manage it.
Oh man, I wish I'd read about this sooner. I didn't realize it was such a drain on ferritin.
I've never had elevated blood markers, but just felt it was a good way to keep them in check while doing some good.
Think I'm going to hold off on donating for a bit and then limit it to just a few times every year.
Appreciate the heads up Madman, I never would've known.
smokedsalmon
Member
4 days in. Insomnia building up, and some anxiety. Going to stick with it a bit longer and see how it goes - unfortunately this is pretty much what happens every time I've tried HCG :/
I have recently taken a break from HCG. I was taking 200iu EOD which is the lowest dose that I have tried so far. Although my blood markers were pretty much perfect I was still feeling a little off, with puffy face, excessive sweating and some anxiety. After 2 weeks of sticking with just T only, no HCG and no AI I feel great!
Of course this is far too early to tell and I will do bloods in about 10 days time to see what is going on. Testicles have still maintained size for now but again, far too early to see any affect here.
My hope is that I can find an absolute minimal dose of HCG that keep testicles moving but doesn't cause the inevitable E2 rise requiring the administration of an AI.
Of course this is far too early to tell and I will do bloods in about 10 days time to see what is going on. Testicles have still maintained size for now but again, far too early to see any affect here.
My hope is that I can find an absolute minimal dose of HCG that keep testicles moving but doesn't cause the inevitable E2 rise requiring the administration of an AI.
@albatross and @smokedsalmon how are you diluting your HCG? How much water into how many IU’s? What time of day you inject?
@albatross and @smokedsalmon how are you diluting your HCG? How much water into how many IU’s? What time of day you inject?
For me it is 5ml bac water mixed with a Pregnyl 5000iu vial and inject 200IU EOD first thing in the morning. I think this is pretty standard preparation but my dosage is certainly on the lower end compared to others. I suspect it is still too much for me though.
@albatross I highly recommend You switch to a more concentrated does. That helped me big time. My Endo didn’t haven’t any scientific evidence, only anecdotal, but has seen better results for men to use HCG at a highly concentrated dose. She says when it’s less diluted men seem to be more sensitive to its estrogenic effects. It was true for me. Less bloating/blushing/hot flashes when I started concentrating it more. I do 12k IU’s to 4ml of water. Then inject .1 which is 300IU’s 2x week. That is the minimum dosage I need to maintain testicular volume.
Just a thought, it’s worth a try, worked for me.
Just a thought, it’s worth a try, worked for me.
Correction - when it’s more diluted, men are more sensitive to its estrogenic effects. You want as little water to HCG as possible.
@Executive7 does this stand up to scrutiny? It is just mixed with bac water so I can't fathom why dilution would affect it - is it because it disperses more easily through the body?
@albatross as stated in my post, absolutely no scientific evidence, purely anecdotal, but this is coming from my endocrinologist and her experiencing in treating men with HCG. She has noticed most men react differently depending on dilution ratio. She has noticed a more concentrated does is received better and notes more estrogenic effects when it’s heavily diluted. I can’t think of any reason why this would be scientifically true, maybe at higher concentrated levels the bodies receptors perceive it differently or metabolize it quicker so it ignites a different response. I have no idea, but if one is having issues with HCG then there’s absolutely no harm in trying it. It did work for me.
@Executive7 Thanks for the detailed reply. It is certainly worth a go I guess. I am just waiting for baseline results for the 4 weeks I have been on T only and depending on the results of that I will decide how to proceed.
i have heard this too but cant remember where@albatross as stated in my post, absolutely no scientific evidence, purely anecdotal, but this is coming from my endocrinologist and her experiencing in treating men with HCG. She has noticed most men react differently depending on dilution ratio. She has noticed a more concentrated does is received better and notes more estrogenic effects when it’s heavily diluted. I can’t think of any reason why this would be scientifically true, maybe at higher concentrated levels the bodies receptors perceive it differently or metabolize it quicker so it ignites a different response. I have no idea, but if one is having issues with HCG then there’s absolutely no harm in trying it. It did work for me.
I have been using Dr Rotman for my HCG and it worked well, but do to the COVID thing I didn't want to go in so he has been ordering it for me from Hallendale which is also very goodCurious how this goes for you man. I think I'm just going to go for it, 300IU every other day.
I'm still using the last of my Empower T cyp, and I want to try this before I run out and switch to the brand from Dr Rotman's pharmacy. I've seen way too much fluctation.
what is your hcg dosage and protocol?I have been using Dr Rotman for my HCG and it worked well, but do to the COVID thing I didn't want to go in so he has been ordering it for me from Hallendale which is also very good
whats your latest blood levels showing? are you still on the hcg, if so what protocol?4 days in. Insomnia building up, and some anxiety. Going to stick with it a bit longer and see how it goes - unfortunately this is pretty much what happens every time I've tried HCG :/
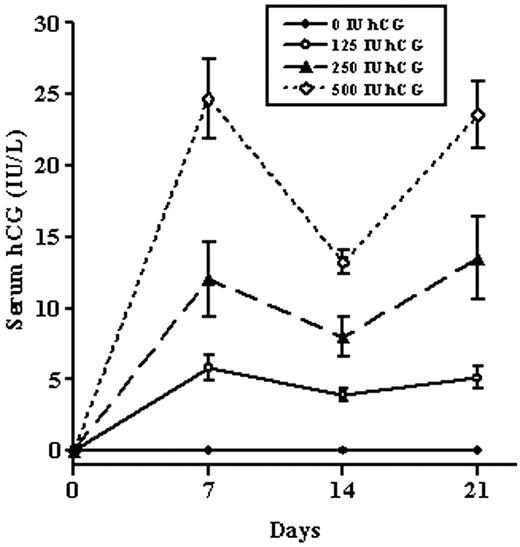
Low-Dose Human Chorionic Gonadotropin Maintains Intratesticular Testosterone in Normal Men with Testosterone-Induced Gonadotropin Suppression
In previous studies of testicular biopsy tissue from healthy men, intratesticular testosterone (ITT) has been shown to be much higher than serum testosterone (Tacademic.oup.com
Discussion
A significant intratesticular fluid to serum T gradient was observed in this group of young normal men at baseline. In this study, serum T was 1.2% of ITT, an 84-fold gradient. A similar testicular to serum gradient has been reported in studies of testicular biopsy tissue in the 1970s (19) as well as more recently (9, 13). However, the absolute ITT levels reported in testicular homogenates are higher than the ITT levels found in the testicular fluid aspirates in this study. This difference is probably the result of the release of cellular T stores in testicular homogenates compared with secreted T in fluid aspirates obtained with minimal cellular disruption. Normal intratesticular fluid T concentrations were maintained by low doses of hCG (125, 250, and 500 IU every other day for 3 wk) in men with gonadotropin suppression from exogenous T. Presumably, normal ITT levels within the testis should support normal spermatogenesis.
madman, thank you for posting these studies. it stated in one 'Furthermore, we have demonstrated that very low-level LH-like stimulation of the testes with hCG increases IT-T in a dose-dependent manner. Importantly, our results suggest that the threshold dose for stimulating IT-T in humans is likely to lie between 15 and 60 IU of hCG.
does this also hold true for men doing trt with hcg? They stated that they thought men using hcg alone where more responsive to it, just wondering if trt further reduced responsiveness hence high dose hcg needed for them?
As expected, we observed that serum gonadotropin levels were significantly reduced by exogenous T in this study. Gonadotropin suppression without hCG administration caused dramatic reductions in ITT (94%) from baseline in the TE and placebo hCG group. Exogenous TE (200 mg weekly) has also been shown to reduce sperm production to azoospermic levels in approximately 70% of Caucasian men (7, 8). Spermatogenesis was not assessed in this 3-wk study, but in a previous study of normal men (n 7) with gonadotropin suppression induced with 6 months of T and a progestin, levonorgestrel (LNG), intratesticular fluid T was suppressed 98% from baseline (15).
In this study, hCG increased the ITT concentration, presumably through stimulation of Leydig cell steroidogenesis. The dose of hCG required to maintain baseline ITT concentrations in men with maximal gonadotropin suppression is significantly lower than that historically used in the treatment of infertility due to hypogonadotropic hypogonadism.
A review of the literature reveals a broad range of relatively high doses of gonadotropin replacement using hCG ranging from 1250 IU three times weekly to 3000 IU twice weekly (29 –32). Even higher doses of hCG (5000 IU, three times per week) have been shown to be safe in experimental models of gonadotropin withdrawal (33, 34). Regimens of 2000 IU administered im two or three times weekly have been used with hCG dose adjustment according to serum T levels with a goal of normal physiological serum T levels (32, 35, 36). This approach is based on the assumption that if normal serum T levels were established by hCG administration, ITT concentrations would be sufficient to support normal spermatogenesis. However, ITT was never directly assessed in these studies. The minimum hCG dose needed to restore ITT to levels sufficient for initiating and maintaining spermatogenesis is not known.
All three hCG groups in this study (125, 250, and 500 IU, given every other day) maintained ITT at levels statistically indistinguishable from the baseline. These doses are 10–20% of the doses commonly used in male infertility treatment (1250–2000 IU, two or three times weekly). Endocrinologists and andrologists have been aware that the doses of hCG traditionally used to treat certain types of infertility are supraphysiological and may expose patients to high levels of T and estradiol, with the consequent risk of clinically significant gynecomastia (37). The ability to prescribe hCG doses at lower levels to target normal serum and ITT and normal spermatogenesis would be useful for this patient population.
However, men rendered hypogonadotropic with exogenous T administration are different from men with infertility due to hypogonadotropic hypogonadism in two important ways. First, the study participants started with normal gonadotropin levels and were treated with high dose TE to induce gonadotropin withdrawal at the same time they were treated with hCG with the aim of maintaining ITT. In contrast, hypogonadotropic infertile men are treated with either T replacement or hCG for fertility, but not both simultaneously. The weekly administration of TE raised serum T levels significantly in all groups and may have resulted in higher ITT concentrations than would have been observed in a patient with hypogonadotropic hypogonadism receiving hCG therapy alone. Second, in the clinical setting, ITT production and spermatogenesis have to be induced after a prolonged period of gonadotropin deficiency. Therefore, the low-dose hCG used in this study may not normalize ITT in hypogonadotropic infertile men. However, lower hCG doses than those traditionally used may be sufficient to restore spermatogenesis.
In summary, assessment of the testicular hormonal environment through percutaneous fluid aspiration has shown a similar testis to serum T gradient as previous testicular biopsy studies in men and rats. Additionally, low doses of hCG maintain baseline levels of ITT in men with gonadotropin withdrawal from exogenous T administration. Lower doses of hCG may be as effective in treating male infertility due to hypogonadotropism as the higher doses used historically. Selective replacement of LH activity with low-dose hCG, as demonstrated in this study, will allow the design of future studies investigating the relative roles of intratesticular androgens and FSH in the control of human spermatogenesis. Such work will be applicable to the goal of developing uniformly effective male contraception.

Dose-Dependent Increase in Intratesticular Testosterone by Very Low-Dose Human Chorionic Gonadotropin in Normal Men with Experimental Gonadotropin Deficiency
Context and Objective: In men with infertility secondary to gonadotropin deficiency, treatment with relatively high dosages of human chorionic gonadotropinacademic.oup.com
Discussion
In the study, we used testicular aspiration, coupled with gonadotropin suppression, and graded, low doses of hCG to determine the dose-response relationship between intratesticular androgens and hCG in man. This study is the first to examine the relationship of such low doses of hCG with intratesticular androgens and to correlate the concentrations of intratesticular androgens with contemporaneously measured serum hormones. Interestingly, we have shown that IT-T concentrations remain much higher than serum testosterone concentrations despite marked LH suppression. Furthermore, we have demonstrated that very low-level LH-like stimulation of the testes with hCG increases IT-T in a dose-dependent manner. Importantly, our results suggest that the threshold dose for stimulating IT-T in humans is likely to lie between 15 and 60 IU of hCG. The measurement of IT-T, coupled with sensitive and specific liquid chromatography-tandem mass spectrometry hormone measurements, and longer-term low-dose gonadotropin administration in this experimental gonadotropin-deficient human model will permit more detailed investigation of the hormonal regulation of spermatogenesis in man than previously possible.
Normal men appear to be more sensitive to hCG than infertile men with hypogonadotropic hypogonadism. This difference in sensitivity is likely due to the fact that steroidogenesis in men with long-term gonadotropin deficiency is impaired, possibly secondary to Leydig cell immaturity. A similar phenomenon has been observed in the hpg mouse, in which larger doses of gonadotropins are required to initiate spermatogenesis than to maintain it once established (19). Our previous work in this area, which used doses of hCG closer to those used in hypogonadotropic infertile men, resulted in IT-T concentrations that were not significantly lower than normal (15). Therefore, in this study, we chose very low doses of hCG to better understand the full dose-response relationship. Notably, in this study, we found that having normal serum testosterone while receiving hCG does not correspond to an IT-T concentration similar to those observed at baseline. The implications of this for the induction of spermatogenesis in men with hypogonadotropic hypogonadism are unknown. However, it is possible that the observation that serum hCG is highly correlated with IT-T may prove useful in the treatment of men with infertility from gonadotropin deficiency. As a result, clinicians may consider measuring both serum testosterone and serum hCG to ensure the adequacy of treatment; however, future studies of the relationship between serum hCG and IT-T in men with hypogonadotropic hypogonadism will be required to determine the utility of this measurement.
In conclusion, this study demonstrates the strong dose-response relationship between IT-T and very low-dose hCG administration in gonadotropin-suppressed men. This work provides crucial information for future studies determining the role of intratesticular androgens on spermatogenesis in men and may improve the treatment of men with infertility and inform efforts to develop male hormonal contraceptives.
hCG Mixing Calculator
HCG Mixing Protocol Calculator
Similar threads
- Replies
- 8
- Views
- 2K
- Replies
- 6
- Views
- 1K
- Replies
- 9
- Views
- 2K
- Replies
- 23
- Views
- 2K
TRT Hormone Predictor
Predict estradiol, DHT, and free testosterone levels based on total testosterone
⚠️ Medical Disclaimer
This tool provides predictions based on statistical models and should NOT replace professional medical advice. Always consult with your healthcare provider before making any changes to your TRT protocol.
ℹ️ Input Parameters
Normal range: 300-1000 ng/dL
Predicted Hormone Levels
Enter your total testosterone value to see predictions
Results will appear here after calculation
Understanding Your Hormones
Estradiol (E2)
A form of estrogen produced from testosterone. Important for bone health, mood, and libido. Too high can cause side effects; too low can affect well-being.
DHT
Dihydrotestosterone is a potent androgen derived from testosterone. Affects hair growth, prostate health, and masculinization effects.
Free Testosterone
The biologically active form of testosterone not bound to proteins. Directly available for cellular uptake and biological effects.
Scientific Reference
Lakshman KM, Kaplan B, Travison TG, Basaria S, Knapp PE, Singh AB, LaValley MP, Mazer NA, Bhasin S. The effects of injected testosterone dose and age on the conversion of testosterone to estradiol and dihydrotestosterone in young and older men. J Clin Endocrinol Metab. 2010 Aug;95(8):3955-64.
DOI: 10.1210/jc.2010-0102 | PMID: 20534765 | PMCID: PMC2913038
Online statistics
- Members online
- 6
- Guests online
- 234
- Total visitors
- 240
Totals may include hidden visitors.
Latest posts
-
-
-
-
The doctor who overturned medicine’s fear of testosterone
- Latest: Nelson Vergel
-
-
-
-












