madman
Super Moderator
The Potential for Pharmacological Interventions for Low Sex Drive in Men (2021)
Amit G. Reddy, MD,1 Amelia A. Khoei, BS,2 and Mohit Khera, MD1
NTRODUCTION
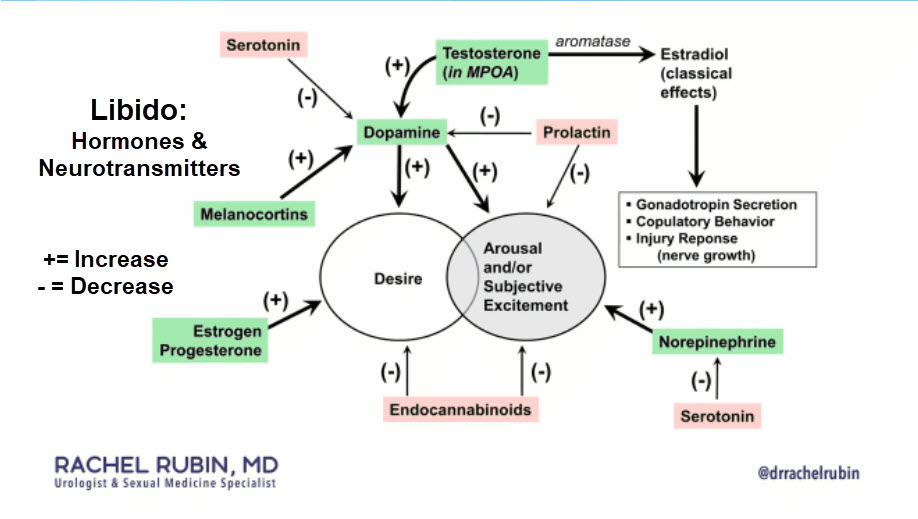
Despite numerous advancements in the field of sexual medicine, there remain a handful of conditions that are difficult to characterize, have poorly understood pathophysiology, and are challenging to treat. Of this subset of pathologies, a complaint of decreased libido or low sex drive (LSD) is one that is familiar to most sexual medicine providers. The reported prevalence of LSD in the male population ranges from as low as 5% to greater than 17%.1,2 The variability in these reported rates is likely due to a combination of factors including a lack of universal diagnostic guidelines for identifying LSD and the societal stigma associated with sexual dysfunction leading many men to repress these issues. Moreover, a cultural component also exists as the discussion of issues pertaining to sex is considered a private affair and can be taboo in many parts of the world. While various definitions of LSD exist in the literature, in the broadest context, it refers to a recurrent absence of sexual desires or fantasies, which causes personal distress and impacts an individual’s quality of life or wellbeing. Furthermore, irrespective of the definition used for LSD, there is a universally strong emphasis that the lack of sexual drive or libido must cause patient distress in order to be considered pathologic and demand intervention. In the Diagnostic and Statistical Manual of Mental Disorders (DSM-V), a condition termed male hypoactive sexual desire disorder (HSDD), is used to describe patients with low libido or sex drive that is independent and not a clinical sequela of other psychiatric or endocrinologic conditions, or due to pharmacologic side effects.3 Additionally, there is also a distinction that the diagnosis of HSDD cannot be made if a comorbid sexual dysfunction exists. Given the strong prevalence of comorbid sexual dysfunction in men presenting with signs of low libido, Rubio-Aurioles et al. make the case that the term HSDD should then refer only to men without any confounding causes for decreased libido or sex drive as outlined by the DSM-V, and LSD should be a broader term that encompasses men with comorbid conditions, including other sexual dysfunctions, that could contribute to a diminished sexual desire.4 For the purposes of this article, this distinction will be used.
DIAGNOSTIC CRITERIA AND WORK-UP
When a man presents with signs and symptoms of low libido, it is important, to begin with, a thorough history and physical examination. The purpose of this evaluation is to first identify comorbid conditions that could be causative factors for his symptoms. It is best to approach the work-up in a systematic manner with a systems-based approach. In the case of psychiatric illnesses, depression, post-traumatic stress disorder, and schizophrenia are just a few of the conditions that can lead to low libido and must be screened for.5 Furthermore, as part of this phase of the work-up, interpersonal relationship stressors and side effects from psychotropic medications must be considered. From a sexual health standpoint, screening for erectile dysfunction, premature ejaculation, and other disorders of sexual function is also key as such conditions are frequently present in this population and may mask low libido.6 Next, an endocrinologic evaluation with serum testosterone, thyroid-stimulating hormone (TSH), and prolactin levels should be undertaken. Finally, if all of these screening and diagnostic tests are negative, a true diagnosis of HSDD must be considered. While this may seem like a straightforward and algorithmic approach (Figure 1), it is important to realize that in any clinical case, a man may be suffering from one or more of the aforementioned conditions, and it can be quite challenging to delineate if his low libido is the primary pathology or a clinical sequela of another condition.
CURRENT PHARMACOLOGIC THERAPIES
There are currently a limited number of pharmacologic options that are actively being investigated for men diagnosed with HSDD. In the case of patients with LSD, the treatment plan often revolves around treating the underlying comorbid condition that may be triggering low libido. For example, in a patient suffering from hypothyroidism, the treatment would focus on restoring hormonal balance and subsequently reassessing libido. If the patient still reports distress due to persistent low libido, a diagnosis of HSDD can subsequently be considered and the patient can be treated as such. From an epidemiologic standpoint, given that HSDD is more prevalent in females, a vast majority of the currently available data and therapies focus on female patient populations. As a result, a great deal of the current clinical treatment landscape for male HSDD consists of off-label use of therapeutics, often with minimal published data to support their use (Table 1). It should also be noted that sex therapy has also been a proven modality of treatment in the HSDD population. However, further details regarding this type of treatment are outside the scope of this review.
The use of many of these therapeutics remains an area of active research, and as a result, minimal data exists on the long-term efficacy and safety of these medications. Additionally, protocols to safely discontinue or wean these treatments do not exist. However, for patients who experience adverse effects or have therapeutic failure and wish to discontinue treatment, it would be prudent to attempt a taper-based method of discontinuation given that many of these therapies are hormone-based
*TESTOSTERONE AND DEHYDROEPIANDROSTERONE
Testosterone and its derivatives are some of the most commonly prescribed medications for men with low libido. Guidelines set forth by many academic governing bodies such as the Endocrine Society have shown through systematic review and meta-analysis data that clinically significant improvements in low libido are only seen in hypogonadal men who are treated with testosterone replacement therapy, and no such improvements are noted in their eugonadal counterparts suffering from these symptoms.7 In eugonadal men with low libido, a comprehensive workup as previously described should be undertaken to identify other etiologies for their symptoms. Unfortunately, off-label use of testosterone replacement therapies for the treatment of low libido in eugonadal men has become all too common in the community practice setting and can not only be ineffective for patients but also have significant risk for short and long-term side effects.
Another androgen therapy that has been investigated as a treatment for low libido is the naturally occurring steroid hormone precursor Dehydroepiandrosterone (DHEA). In the central nervous system, the prohormone acts as a neurosteroid and has excitatory effects on neuronal transmission. Peripherally, the prohormone is converted to one of many hormones, including testosterone, and has a multitude of downstream effects on components of sexual function including libido and arousal. The differences in how exogenous DHEA and its derivatives impact sexual function in men and women suffering from HSDD and other disorders of sexual function are still under investigation. In the current literature, the efficacy of DHEA in the treatment of HSDD has demonstrated mixed results in men and women. In a more recent double-blind, placebo-controlled short-term study utilizing a higher dosage regiment (100 mg daily) than prior studies, significant improvements were seen in arousal in postmenopausal women with HSDD, while no effects were noted in men with HSDD.8 Given that arousal and desire are closely linked components of sexual function, the authors claim that despite a lack of objective improvement in desire, such increases in arousal may indirectly improve desire. Additionally, the authors report that exogenous DHEA administration increased central and peripheral androgen levels, and specifically, hypothesized that the mechanism through which DHEA mediates its effects on sexual function is through increased serum testosterone levels. This would explain why eugonadal men in this study did not show any significant clinical improvement, as increased testosterone levels in this population have not been shown to positively impact libido as previously described. While the authors argue that an even higher dosage of DHEA may demonstrate positive results in men, if in fact, DHEA mediates its effects through raised serum testosterone levels, then it is unlikely that higher doses would yield any improvements.
*DOPAMINE AGONISTS
Bupropion (Wellbutrin, Aplenzin, Zyban) is an FDA-approved anti-depressant medication that is used to treat adult depression and seasonal affective disorder and can assist with smoking cessation.9 Despite its use for the treatment of these conditions and other off-label indications, bupropion’s mechanism of action has still not been fully elucidated. Current data suggests that its effects are mediated through inhibition of norepinephrine and dopamine reuptake. While multiple recent studies have explored the use of bupropion in the treatment of female HSDD populations, minimal literature exists regarding buproprion’s effectiveness in men.10 Of note, in 1987, Crenshaw et al. studied buproprion’s effects through a double-blind trial in 30 men and women with some form of psychosexual dysfunction (inhibited sexual desire, sexual excitement, and orgasm).11 Importantly, participants were screened for underlying psychiatric or organic etiologies for their symptoms and excluded from the trial if other possible causative factors were found. After a 12- week trial period, participants in the treatment arm reported a statistically significant improvement in libido and sexual function with 63% of the group’s participants reporting improvement in these areas in comparison to just 3% of the placebo group. Additionally, the improvement was more prominent amongst the male cohort, with 86% of men showing improvement in comparison to 44% of women. Apart from mild and previously known side effects such as weight loss, nausea, agitation, and insomnia, no other major side effects were reported in the treatment group. While the results in this study showed promise, no subsequent data regarding the use of bupropion for men suffering from HSDD currently exists and at present, its use for this indication remains off-label. Recently, a combination agent, consisting of buproprion and trazadone (Lorexys), was tested in an open-label 1-way crossover design for female HSDD and demonstrated superior results in comparison to a bupropion alone group.12 Clearly, further studies attempting to understand the mechanism of action, optimal dosing regimens, and potential long-term side effects will be key to establishing bupropion and medications like it, as potential therapies for the treatment of HSDD in the male population.
*SEROTONIN RECEPTOR MODULATORS
Flibanserin (Addyi) is a novel therapy for the treatment of female HSDD. After achieving FDA approval in 2015, the drug was marketed for premenopausal women suffering from HSDD of any severity. Currently, it is theorized that flibanserin acts as an agonist at the 5HT1A post-synaptic receptor and as an antagonist at the 5HT2A post-synaptic receptor. Through modulation of these post-synaptic serotonin receptors in the pre-frontal cortex, flibanserin mediates the release of dopamine and norepinephrine, which play an important role in promoting sexual excitation.13 Concurrently, flibanserin also reduces the level of serotonin, which normally reduces sexual interest by inhibiting dopamine release. At present, flibanserin is not approved for the treatment of low libido in men. While it is theoretically feasible that similar pathophysiology for HSDD exists in men as in women, clinical trial data would be necessary to prove the effectiveness and safety of flibanserin in men before any widespread off-label use can be recommended. At present, it is prudent for all prescribers of flibanserin to educate patients on potential side effects including risks for hypotension and syncope, and avoiding the concurrent use of cytochrome P450 3A4 (CYP3A4) inhibitors.13
*MELANOCORTINS
Melanocortins are another drug class with the potential to be pharmacologic treatment options for patients with HSDD. In 2019, the FDA approved the bremelanotide therapeutic (Vyleesi) for the treatment of HSDD in premenopausal women. Early evidence suggests that bremelanotide, an analog of the neuropeptide a-melanocyte-stimulating hormone, acts on a multitude of melanocortin receptors that are involved in excitatory pathways that have been implicated in sexual desire and arousal.14 Similar to flibanserin, bremelanotide’s mechanism of action in improving sexual desire has still not been fully elucidated and more pre-clinical data will be necessary to better characterize these neural pathways. In addition, even less information exists regarding bremelanotide’s safety and efficacy in improving low sex drive in male HSDD patients. However, bremelanotide has been shown in pre-clinical and clinical studies to improve erectile function in men through stimulation of centrally-acting melanocortin receptors located in the hypothalamus, and some data suggest that activation of this pathway also may trigger oxytocin release that may be implicated in sexual function.15,16 Future investigation into bremelanotide may provide more information regarding its role in the treatment of low libido in men.
CONCLUSIONS
The treatment of low libido in HSDD men remains an area of active research and development. While numerous compounds and repurposed therapeutics have been investigated, only a finite number have gained widespread consideration among sexual medicine providers. One of the major barriers to improved therapeutics in this area likely stems from the fact that the pathophysiology of HSDD still remains poorly understood and as a result, molecular targets for drug development have been challenging to identify. It is evident that going forward, more pre-clinical and clinical data focused on further elucidating the pathophysiology of HSDD will be crucial to uncovering more effective therapeutics for this patient population.
Amit G. Reddy, MD,1 Amelia A. Khoei, BS,2 and Mohit Khera, MD1
NTRODUCTION
Despite numerous advancements in the field of sexual medicine, there remain a handful of conditions that are difficult to characterize, have poorly understood pathophysiology, and are challenging to treat. Of this subset of pathologies, a complaint of decreased libido or low sex drive (LSD) is one that is familiar to most sexual medicine providers. The reported prevalence of LSD in the male population ranges from as low as 5% to greater than 17%.1,2 The variability in these reported rates is likely due to a combination of factors including a lack of universal diagnostic guidelines for identifying LSD and the societal stigma associated with sexual dysfunction leading many men to repress these issues. Moreover, a cultural component also exists as the discussion of issues pertaining to sex is considered a private affair and can be taboo in many parts of the world. While various definitions of LSD exist in the literature, in the broadest context, it refers to a recurrent absence of sexual desires or fantasies, which causes personal distress and impacts an individual’s quality of life or wellbeing. Furthermore, irrespective of the definition used for LSD, there is a universally strong emphasis that the lack of sexual drive or libido must cause patient distress in order to be considered pathologic and demand intervention. In the Diagnostic and Statistical Manual of Mental Disorders (DSM-V), a condition termed male hypoactive sexual desire disorder (HSDD), is used to describe patients with low libido or sex drive that is independent and not a clinical sequela of other psychiatric or endocrinologic conditions, or due to pharmacologic side effects.3 Additionally, there is also a distinction that the diagnosis of HSDD cannot be made if a comorbid sexual dysfunction exists. Given the strong prevalence of comorbid sexual dysfunction in men presenting with signs of low libido, Rubio-Aurioles et al. make the case that the term HSDD should then refer only to men without any confounding causes for decreased libido or sex drive as outlined by the DSM-V, and LSD should be a broader term that encompasses men with comorbid conditions, including other sexual dysfunctions, that could contribute to a diminished sexual desire.4 For the purposes of this article, this distinction will be used.
DIAGNOSTIC CRITERIA AND WORK-UP
When a man presents with signs and symptoms of low libido, it is important, to begin with, a thorough history and physical examination. The purpose of this evaluation is to first identify comorbid conditions that could be causative factors for his symptoms. It is best to approach the work-up in a systematic manner with a systems-based approach. In the case of psychiatric illnesses, depression, post-traumatic stress disorder, and schizophrenia are just a few of the conditions that can lead to low libido and must be screened for.5 Furthermore, as part of this phase of the work-up, interpersonal relationship stressors and side effects from psychotropic medications must be considered. From a sexual health standpoint, screening for erectile dysfunction, premature ejaculation, and other disorders of sexual function is also key as such conditions are frequently present in this population and may mask low libido.6 Next, an endocrinologic evaluation with serum testosterone, thyroid-stimulating hormone (TSH), and prolactin levels should be undertaken. Finally, if all of these screening and diagnostic tests are negative, a true diagnosis of HSDD must be considered. While this may seem like a straightforward and algorithmic approach (Figure 1), it is important to realize that in any clinical case, a man may be suffering from one or more of the aforementioned conditions, and it can be quite challenging to delineate if his low libido is the primary pathology or a clinical sequela of another condition.
CURRENT PHARMACOLOGIC THERAPIES
There are currently a limited number of pharmacologic options that are actively being investigated for men diagnosed with HSDD. In the case of patients with LSD, the treatment plan often revolves around treating the underlying comorbid condition that may be triggering low libido. For example, in a patient suffering from hypothyroidism, the treatment would focus on restoring hormonal balance and subsequently reassessing libido. If the patient still reports distress due to persistent low libido, a diagnosis of HSDD can subsequently be considered and the patient can be treated as such. From an epidemiologic standpoint, given that HSDD is more prevalent in females, a vast majority of the currently available data and therapies focus on female patient populations. As a result, a great deal of the current clinical treatment landscape for male HSDD consists of off-label use of therapeutics, often with minimal published data to support their use (Table 1). It should also be noted that sex therapy has also been a proven modality of treatment in the HSDD population. However, further details regarding this type of treatment are outside the scope of this review.
The use of many of these therapeutics remains an area of active research, and as a result, minimal data exists on the long-term efficacy and safety of these medications. Additionally, protocols to safely discontinue or wean these treatments do not exist. However, for patients who experience adverse effects or have therapeutic failure and wish to discontinue treatment, it would be prudent to attempt a taper-based method of discontinuation given that many of these therapies are hormone-based
*TESTOSTERONE AND DEHYDROEPIANDROSTERONE
Testosterone and its derivatives are some of the most commonly prescribed medications for men with low libido. Guidelines set forth by many academic governing bodies such as the Endocrine Society have shown through systematic review and meta-analysis data that clinically significant improvements in low libido are only seen in hypogonadal men who are treated with testosterone replacement therapy, and no such improvements are noted in their eugonadal counterparts suffering from these symptoms.7 In eugonadal men with low libido, a comprehensive workup as previously described should be undertaken to identify other etiologies for their symptoms. Unfortunately, off-label use of testosterone replacement therapies for the treatment of low libido in eugonadal men has become all too common in the community practice setting and can not only be ineffective for patients but also have significant risk for short and long-term side effects.
Another androgen therapy that has been investigated as a treatment for low libido is the naturally occurring steroid hormone precursor Dehydroepiandrosterone (DHEA). In the central nervous system, the prohormone acts as a neurosteroid and has excitatory effects on neuronal transmission. Peripherally, the prohormone is converted to one of many hormones, including testosterone, and has a multitude of downstream effects on components of sexual function including libido and arousal. The differences in how exogenous DHEA and its derivatives impact sexual function in men and women suffering from HSDD and other disorders of sexual function are still under investigation. In the current literature, the efficacy of DHEA in the treatment of HSDD has demonstrated mixed results in men and women. In a more recent double-blind, placebo-controlled short-term study utilizing a higher dosage regiment (100 mg daily) than prior studies, significant improvements were seen in arousal in postmenopausal women with HSDD, while no effects were noted in men with HSDD.8 Given that arousal and desire are closely linked components of sexual function, the authors claim that despite a lack of objective improvement in desire, such increases in arousal may indirectly improve desire. Additionally, the authors report that exogenous DHEA administration increased central and peripheral androgen levels, and specifically, hypothesized that the mechanism through which DHEA mediates its effects on sexual function is through increased serum testosterone levels. This would explain why eugonadal men in this study did not show any significant clinical improvement, as increased testosterone levels in this population have not been shown to positively impact libido as previously described. While the authors argue that an even higher dosage of DHEA may demonstrate positive results in men, if in fact, DHEA mediates its effects through raised serum testosterone levels, then it is unlikely that higher doses would yield any improvements.
*DOPAMINE AGONISTS
Bupropion (Wellbutrin, Aplenzin, Zyban) is an FDA-approved anti-depressant medication that is used to treat adult depression and seasonal affective disorder and can assist with smoking cessation.9 Despite its use for the treatment of these conditions and other off-label indications, bupropion’s mechanism of action has still not been fully elucidated. Current data suggests that its effects are mediated through inhibition of norepinephrine and dopamine reuptake. While multiple recent studies have explored the use of bupropion in the treatment of female HSDD populations, minimal literature exists regarding buproprion’s effectiveness in men.10 Of note, in 1987, Crenshaw et al. studied buproprion’s effects through a double-blind trial in 30 men and women with some form of psychosexual dysfunction (inhibited sexual desire, sexual excitement, and orgasm).11 Importantly, participants were screened for underlying psychiatric or organic etiologies for their symptoms and excluded from the trial if other possible causative factors were found. After a 12- week trial period, participants in the treatment arm reported a statistically significant improvement in libido and sexual function with 63% of the group’s participants reporting improvement in these areas in comparison to just 3% of the placebo group. Additionally, the improvement was more prominent amongst the male cohort, with 86% of men showing improvement in comparison to 44% of women. Apart from mild and previously known side effects such as weight loss, nausea, agitation, and insomnia, no other major side effects were reported in the treatment group. While the results in this study showed promise, no subsequent data regarding the use of bupropion for men suffering from HSDD currently exists and at present, its use for this indication remains off-label. Recently, a combination agent, consisting of buproprion and trazadone (Lorexys), was tested in an open-label 1-way crossover design for female HSDD and demonstrated superior results in comparison to a bupropion alone group.12 Clearly, further studies attempting to understand the mechanism of action, optimal dosing regimens, and potential long-term side effects will be key to establishing bupropion and medications like it, as potential therapies for the treatment of HSDD in the male population.
*SEROTONIN RECEPTOR MODULATORS
Flibanserin (Addyi) is a novel therapy for the treatment of female HSDD. After achieving FDA approval in 2015, the drug was marketed for premenopausal women suffering from HSDD of any severity. Currently, it is theorized that flibanserin acts as an agonist at the 5HT1A post-synaptic receptor and as an antagonist at the 5HT2A post-synaptic receptor. Through modulation of these post-synaptic serotonin receptors in the pre-frontal cortex, flibanserin mediates the release of dopamine and norepinephrine, which play an important role in promoting sexual excitation.13 Concurrently, flibanserin also reduces the level of serotonin, which normally reduces sexual interest by inhibiting dopamine release. At present, flibanserin is not approved for the treatment of low libido in men. While it is theoretically feasible that similar pathophysiology for HSDD exists in men as in women, clinical trial data would be necessary to prove the effectiveness and safety of flibanserin in men before any widespread off-label use can be recommended. At present, it is prudent for all prescribers of flibanserin to educate patients on potential side effects including risks for hypotension and syncope, and avoiding the concurrent use of cytochrome P450 3A4 (CYP3A4) inhibitors.13
*MELANOCORTINS
Melanocortins are another drug class with the potential to be pharmacologic treatment options for patients with HSDD. In 2019, the FDA approved the bremelanotide therapeutic (Vyleesi) for the treatment of HSDD in premenopausal women. Early evidence suggests that bremelanotide, an analog of the neuropeptide a-melanocyte-stimulating hormone, acts on a multitude of melanocortin receptors that are involved in excitatory pathways that have been implicated in sexual desire and arousal.14 Similar to flibanserin, bremelanotide’s mechanism of action in improving sexual desire has still not been fully elucidated and more pre-clinical data will be necessary to better characterize these neural pathways. In addition, even less information exists regarding bremelanotide’s safety and efficacy in improving low sex drive in male HSDD patients. However, bremelanotide has been shown in pre-clinical and clinical studies to improve erectile function in men through stimulation of centrally-acting melanocortin receptors located in the hypothalamus, and some data suggest that activation of this pathway also may trigger oxytocin release that may be implicated in sexual function.15,16 Future investigation into bremelanotide may provide more information regarding its role in the treatment of low libido in men.
CONCLUSIONS
The treatment of low libido in HSDD men remains an area of active research and development. While numerous compounds and repurposed therapeutics have been investigated, only a finite number have gained widespread consideration among sexual medicine providers. One of the major barriers to improved therapeutics in this area likely stems from the fact that the pathophysiology of HSDD still remains poorly understood and as a result, molecular targets for drug development have been challenging to identify. It is evident that going forward, more pre-clinical and clinical data focused on further elucidating the pathophysiology of HSDD will be crucial to uncovering more effective therapeutics for this patient population.

















