madman
Super Moderator
Testosterone replacement therapy and cardiovascular risk
Thiago Gagliano-Jucá and Shehzad Basaria
Abstract
Testosterone is the main male sex hormone and is essential for the maintenance of male secondary sexual characteristics and fertility. Androgen deficiency in young men owing to organic disease of the hypothalamus, pituitary gland or testes has been treated with testosterone replacement for decades without reports of increased cardiovascular events. In the past decade, the number of testosterone prescriptions issued for middle-aged or older men with either age related or obesity-related decline in serum testosterone levels has increased exponentially even though these conditions are not approved indications for testosterone therapy. Some retrospective studies and randomized trials have suggested that testosterone replacement therapy increases the risk of cardiovascular disease, which has led the FDA to release a warning statement about the potential cardiovascular risks of testosterone replacement therapy. However, no trials of testosterone replacement therapy published to date were designed or adequately powered to assess cardiovascular events; therefore, the cardiovascular safety of this therapy remains unclear. In this Review, we provide an overview of epidemiological data on the association between serum levels of endogenous testosterone and cardiovascular disease, prescription database studies on the risk of cardiovascular disease in men receiving testosterone therapy, randomized trials and meta-analyses evaluating testosterone replacement therapy and its association with cardiovascular events and mechanistic studies on the effects of testosterone on the cardiovascular system. Our aim is to help clinicians to make informed decisions when considering testosterone replacement therapy in their patients.
Effect of endogenous testosterone level
Epidemiological studies
Summary of epidemiological studies.
In summary, although conflicting results have been obtained in epidemiological studies, most studies suggest that low serum levels of endogenous testosterone are a risk factor for cardiovascular events, cardiovascular mortality and all-cause mortality. However, these data must be interpreted with caution because population studies, no matter how methodologically rigorous, cannot establish causality or exclude reverse causality. Indeed, the researchers in some of these studies suggested that this association is not causal 3,51. Some investigators suggest that testosterone has a beneficial effect on the risk of cardiovascular disease on the basis of studies in men with prostate cancer who received androgen deprivation therapy, which show that low serum testosterone levels are consistently associated with metabolic syndrome, cardiovascular morbidity and mortality 65–69. However, because serum testosterone levels in these men are in the castrate range, which is profoundly lower than the cut-off thresholds in the above-mentioned population studies, these data must also be interpreted with caution.
Table 1 | association between endogenous testosterone levels in serum and cardiovascular events
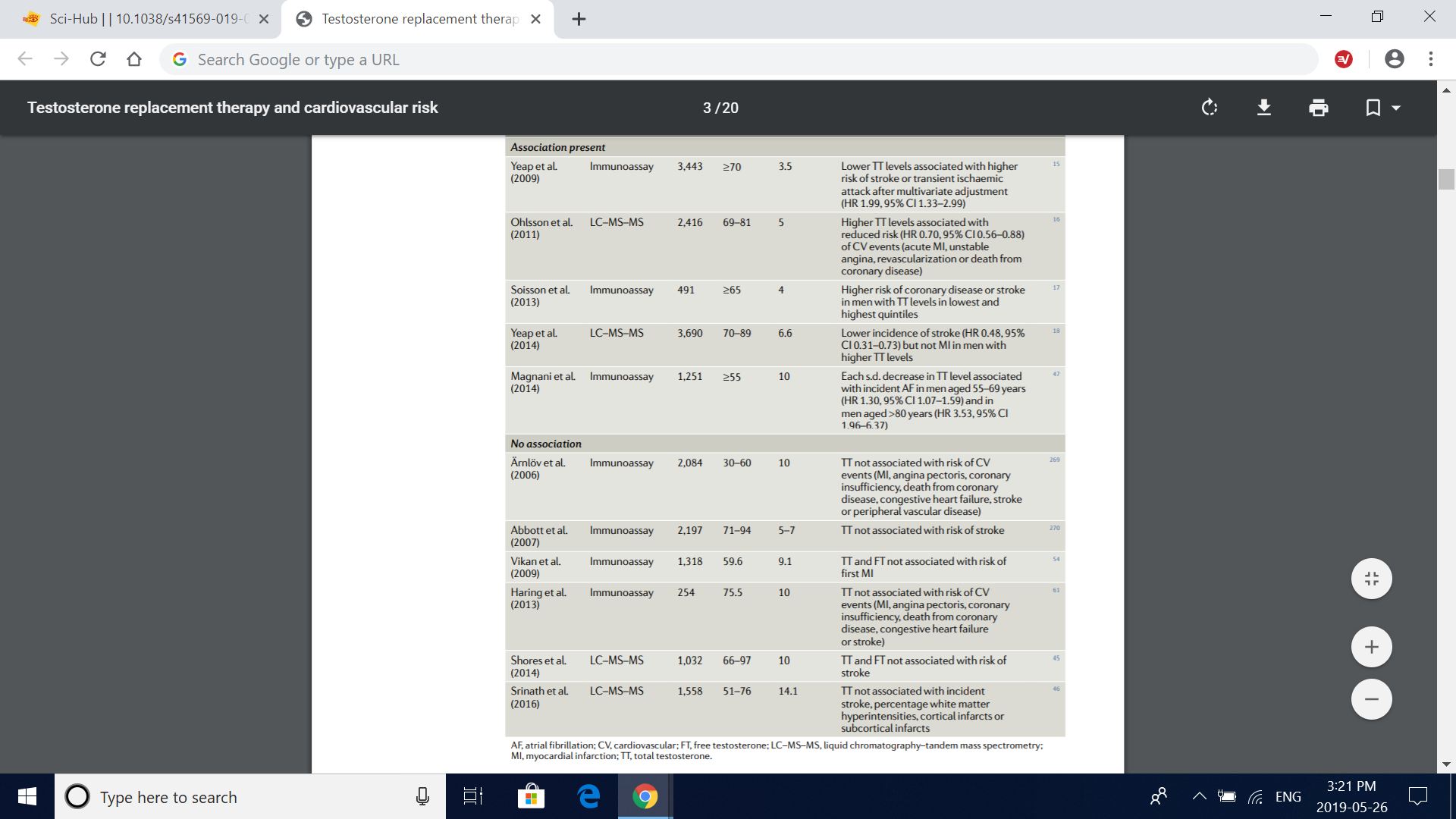
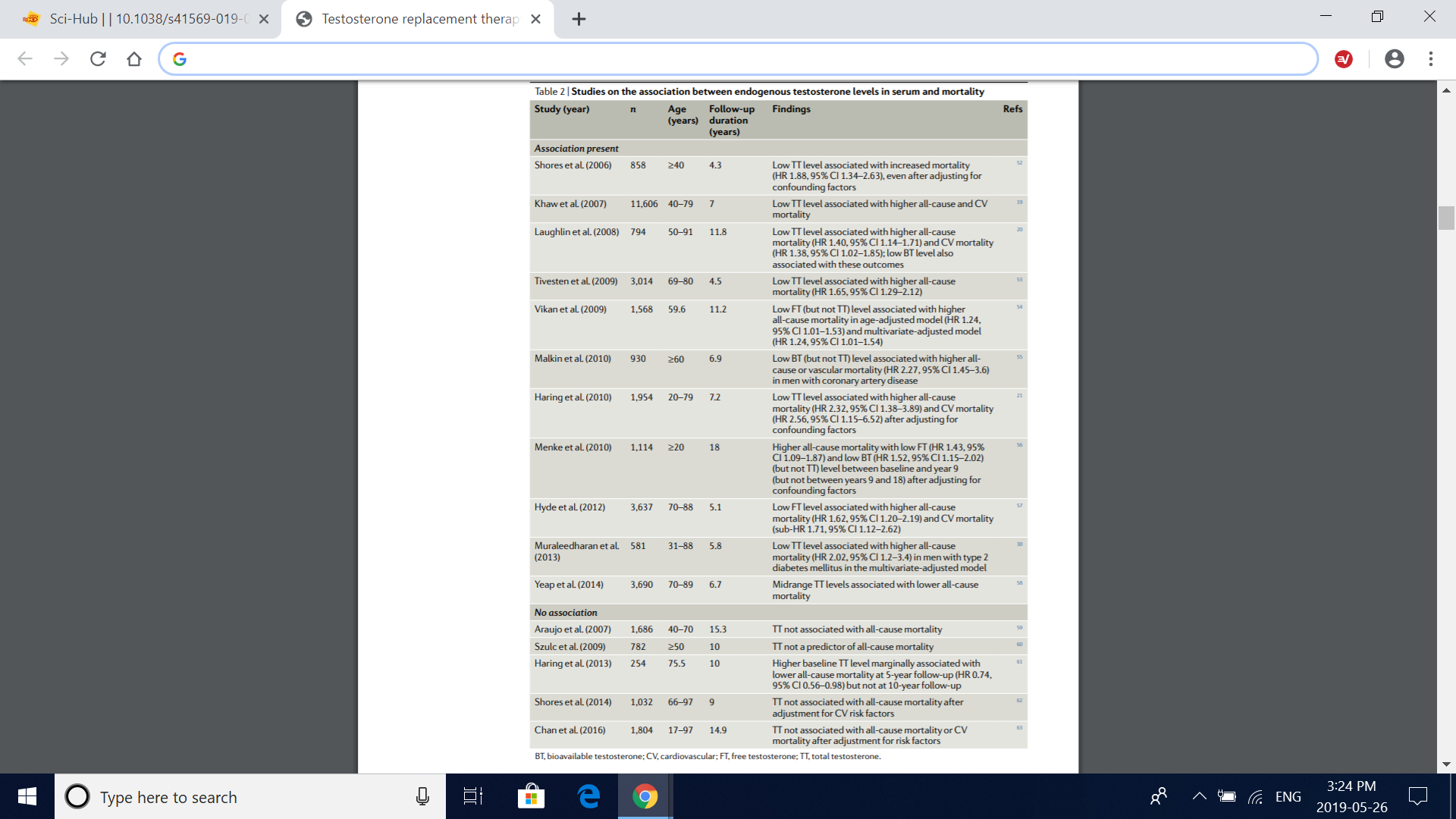
Table 2 | Studies on the association between endogenous testosterone levels in serum and mortality
Effect of testosterone therapy
Retrospective studies
In summary, these retrospective, prescription database studies provide useful information on the cardiovascular effects of testosterone replacement therapy (Table 3). However, because of the inherent design limitations of retrospective studies, making firm conclusions about the cardiovascular safety of testosterone therapy is not possible.
Table 3 | retrospective studies on testosterone replacement therapy and risk of cardiovascular events and death
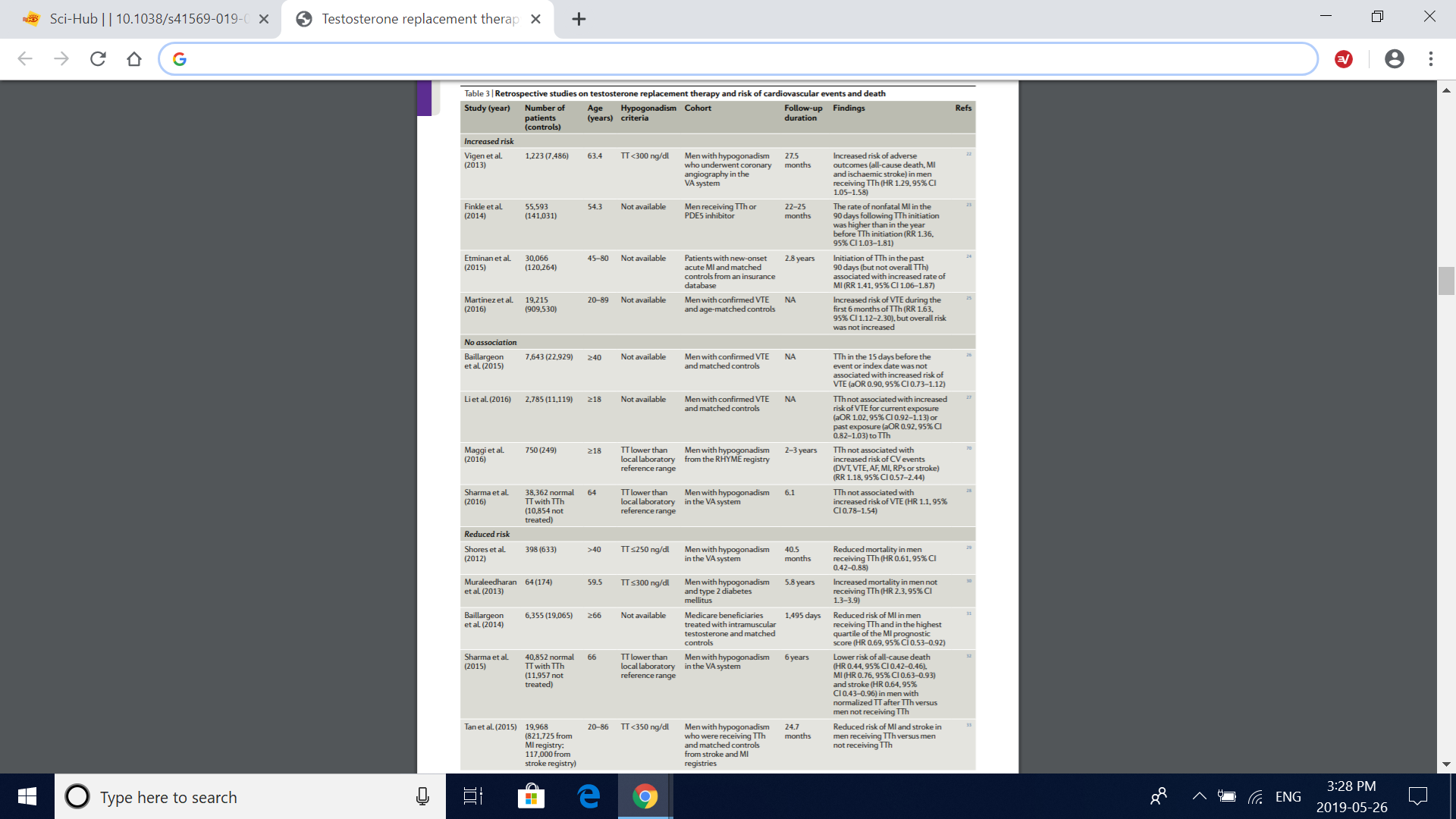
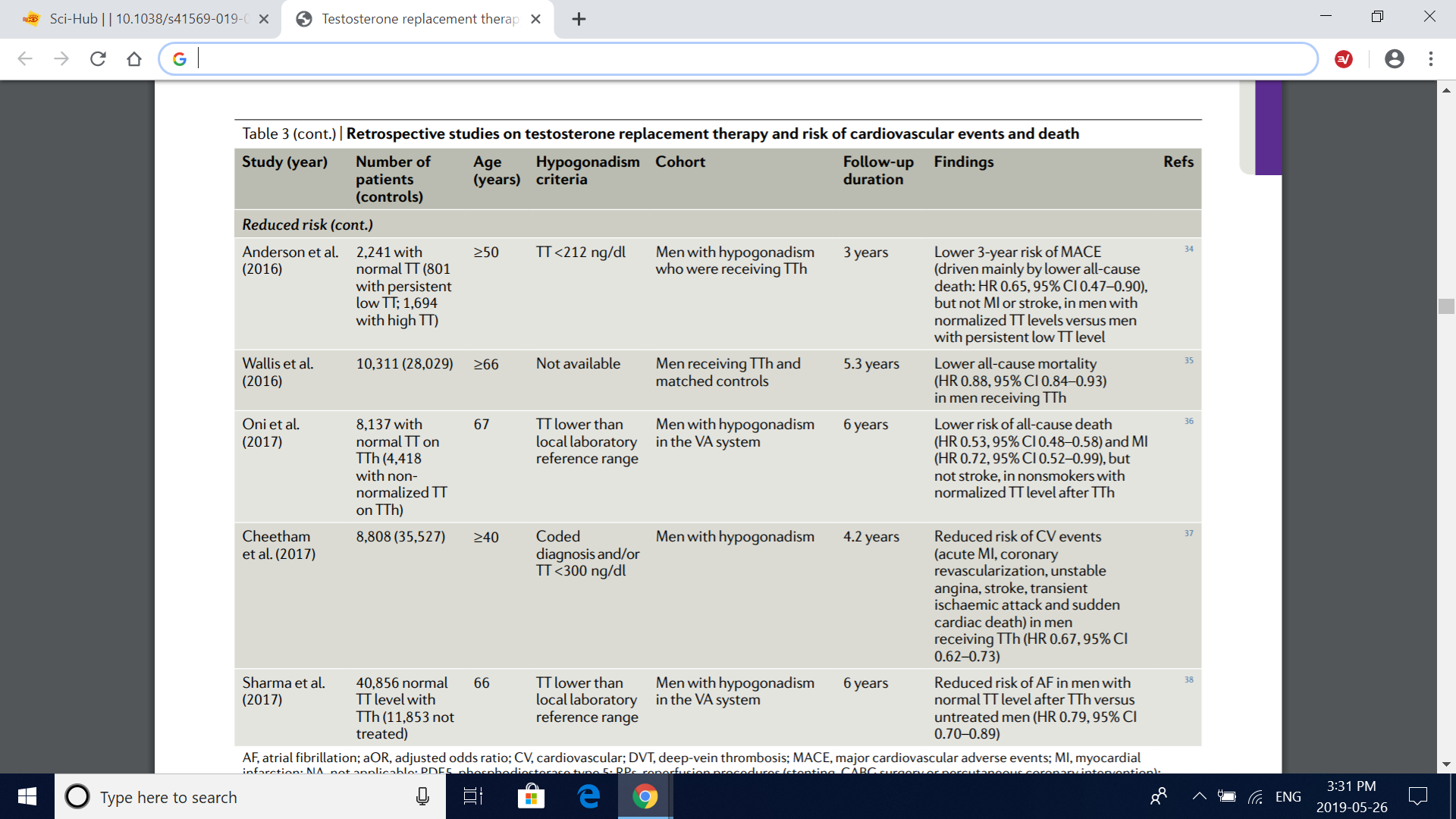
Table 3 (cont.) | retrospective studies on testosterone replacement therapy and risk of cardiovascular events and death
Randomized, controlled trials
The Copenhagen Study Group trial.
The TOM trial.
The TEAAM trial.
The TTrials.
Trials on testosterone as a male contraceptive.
Fig. 2 | Effects of testosterone treatment on coronary artery plaques in clinical trials. a | Changes from baseline in the volume of coronary artery plaques in the cardiovascular substudy of the TTrials79 (CV TTrials). The drawings below the graph depict anatomical representations of the plaque subtypes. Compared with placebo, testosterone treatment for 12 months induced a significant increase in fibrous, soft (noncalcified) and total coronary artery plaque volume, as assessed by coronary CT angiography, whereas no significant treatment effect was seen for fibrous-fatty or calcified coronary artery plaque volume. Bars are mean change from baseline to 12 months with testosterone therapy and placebo adjusted for baseline total testosterone level in the serum (≤200ng/dl or >200ng/dl), age (≤75 years or >75 years), trial site, participation in the main TTrials, use or nonuse of antidepressants and use or nonuse of phosphodiesterase type 5 inhibitors. Error bars are 95% CI. Data are derived from ref. 79. *Significant difference (P<0.05) determined by a linear mixed model with all balancing factors and baseline outcome value as covariates and a random effect for participant. b | Changes from baseline in coronary artery calcium scores in the TEAAM trial77. Compared with baseline, no significant difference was observed between treatment groups in mean estimated yearly change in coronary artery calcium scores, as assessed with multidetector-row CT over 3 years of treatment. Estimates are derived from mixed-effects regression models supplemented by multiple imputation of missing records; 95% CIs were not available. Data are obtained from ref. 77.
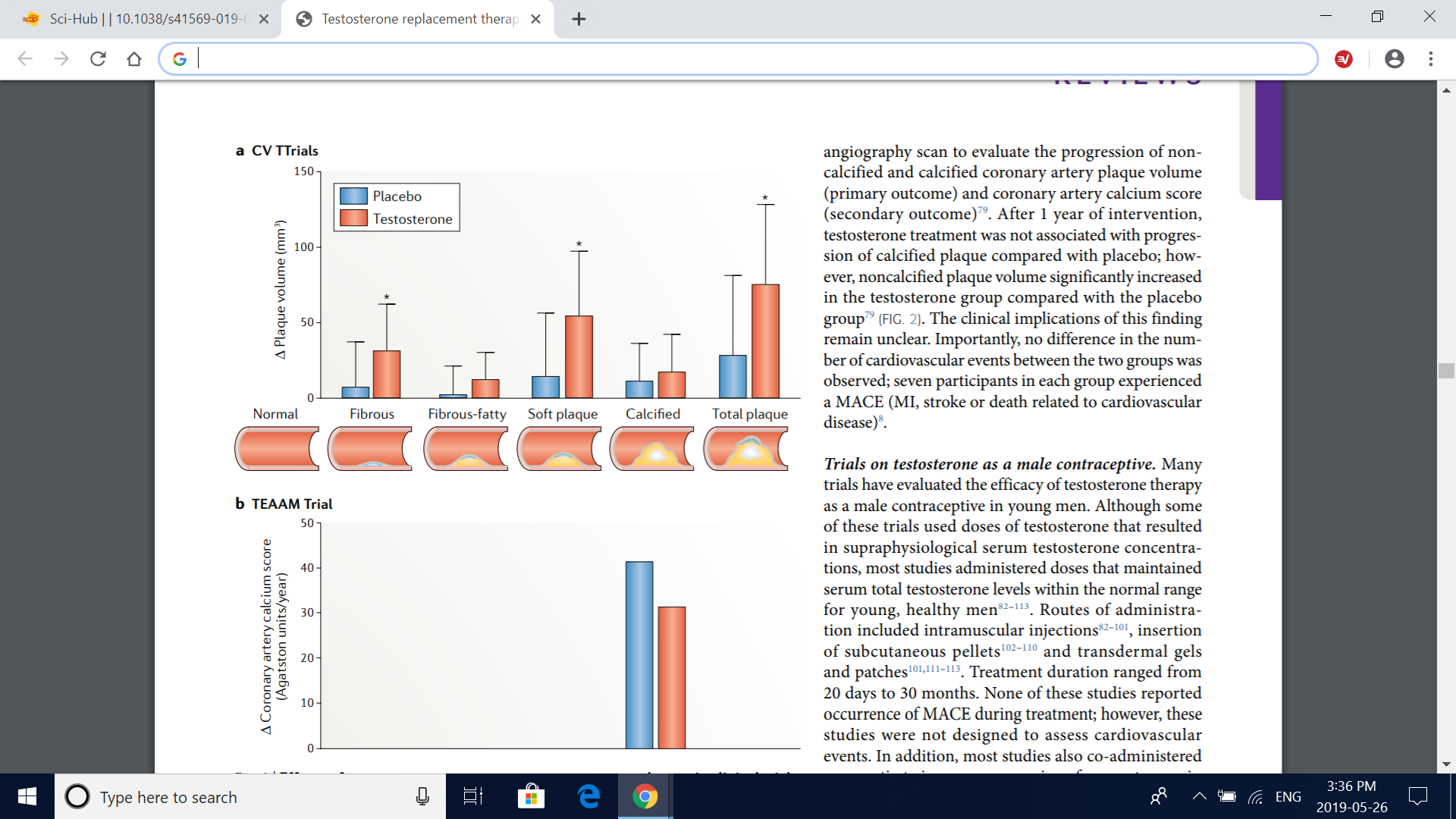
Fig. 3 | Meta-analyses of clinical trials of testosterone replacement therapy. The graph depicts the pooled odds ratio of composite cardiovascular events from metaanalyses of clinical trials of testosterone replacement therapy, with the exception of the study by Alexander et al.118, which reported myocardial infarction (MI) and stroke as separate outcomes. The definition of composite cardiovascular events varied across studies, but all included MI, angina, coronary revascularization and stroke. In addition, the report by Calof et al.114 included atrial fibrillation, sudden death and other vascular events; Haddad et al.115 included cardiovascular death and claudication; FernándezBalsells et al.116 included death, peripheral vascular events, changes in blood lipid fractions, changes in fasting glucose level, new onset of diabetes mellitus and hypertension; Xu et al.40 included any events reported by the researchers as cardiac disorders, cardiovascular complaints, cardiovascular events and vascular disorders; Albert et al.117 included death, syncope, arrhythmia and hospital admission for congestive heart failure; and Corona et al.119 included cardiovascular-related death and heart failure reported as serious adverse event
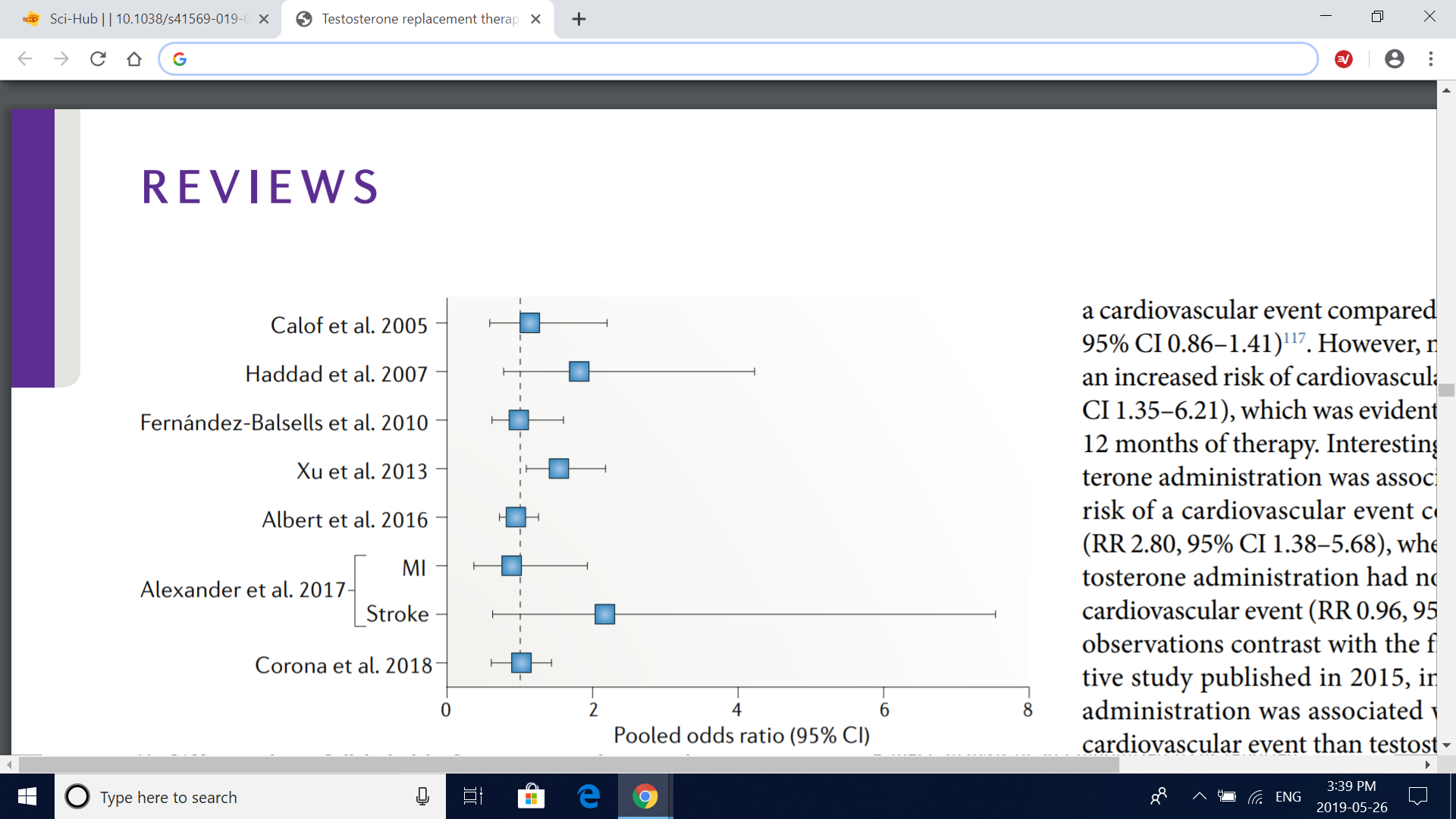
Fig. 4 | Cardiovascular targets and effects of testosterone. Studies in vitro, in animals and in humans have examined the effects of testosterone on various organs and processes. Some studies have shown that testosterone modulates vascular tone, increases erythropoiesis (which can induce erythrocytosis in some men) and affects platelet aggregability and cardiomyocyte electrophysiology and contractile activity. In addition, conflicting findings have been reported in studies evaluating the effect of serum levels of endogenous testosterone and testosterone therapy on atherosclerosis progression and inflammation and on metabolic parameters such as insulin sensitivity and lipid homeostasis; these metabolic effects probably involve complex interactions of the liver, skeletal muscle and adipose tissue.
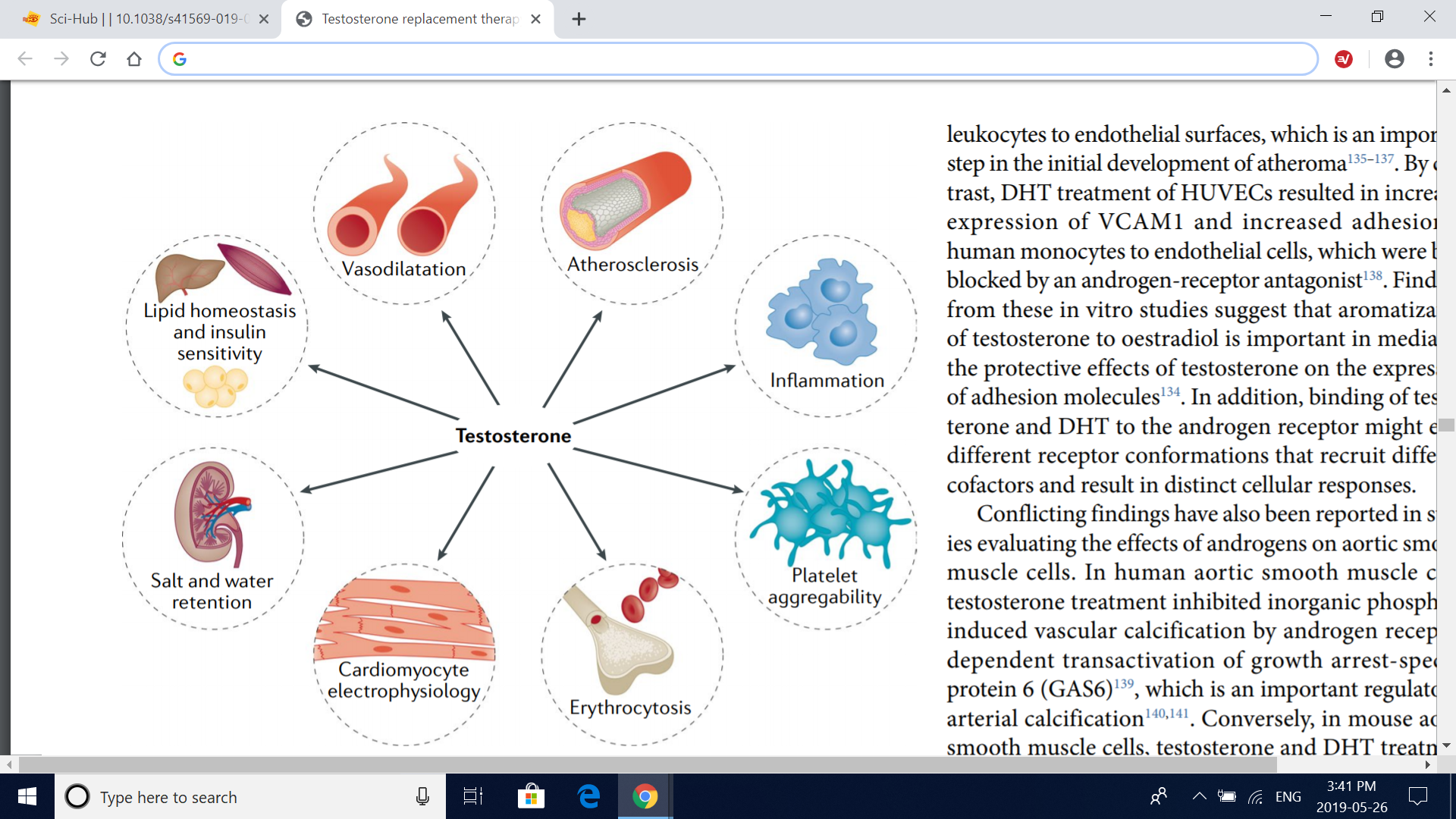
Fig. 5 | Molecular mechanisms of testosterone modulation of vascular tone. Testosterone has effects in both vascular endothelial cells and vascular smooth muscle cells, which together produce changes in vascular tone. Testosterone relaxes the vascular smooth muscle by inhibiting the L-type calcium current through voltagedependent L-type calcium channel subunit α1C (Cav1.2), independently of the vascular endothelium or the androgen receptor (AR). Testosterone also induces vascular smooth muscle relaxation via the opening of big-conductance calcium-activated and voltage-activated potassium channels (BKCa) in vascular smooth muscle cell as well as in endothelial cells. In addition, testosterone induces nitric oxide (NO) synthesis in the endothelial cell by endothelial nitric oxide synthase (eNOS) via an AR-dependent, nontranscriptional mechanism; neuronal nitric oxide synthase (nNOS) in the endothelium might also contribute to the testosterone-induced increase in endothelial NO synthesis. Involvement of small-conductance calcium-activated potassium channels (SKCa) in addition to BKCa in the endothelium-dependent vasodilatory effects of testosterone has also been suggested. RYR, ryanodine receptor; SR, sarcoplasmic reticulum.
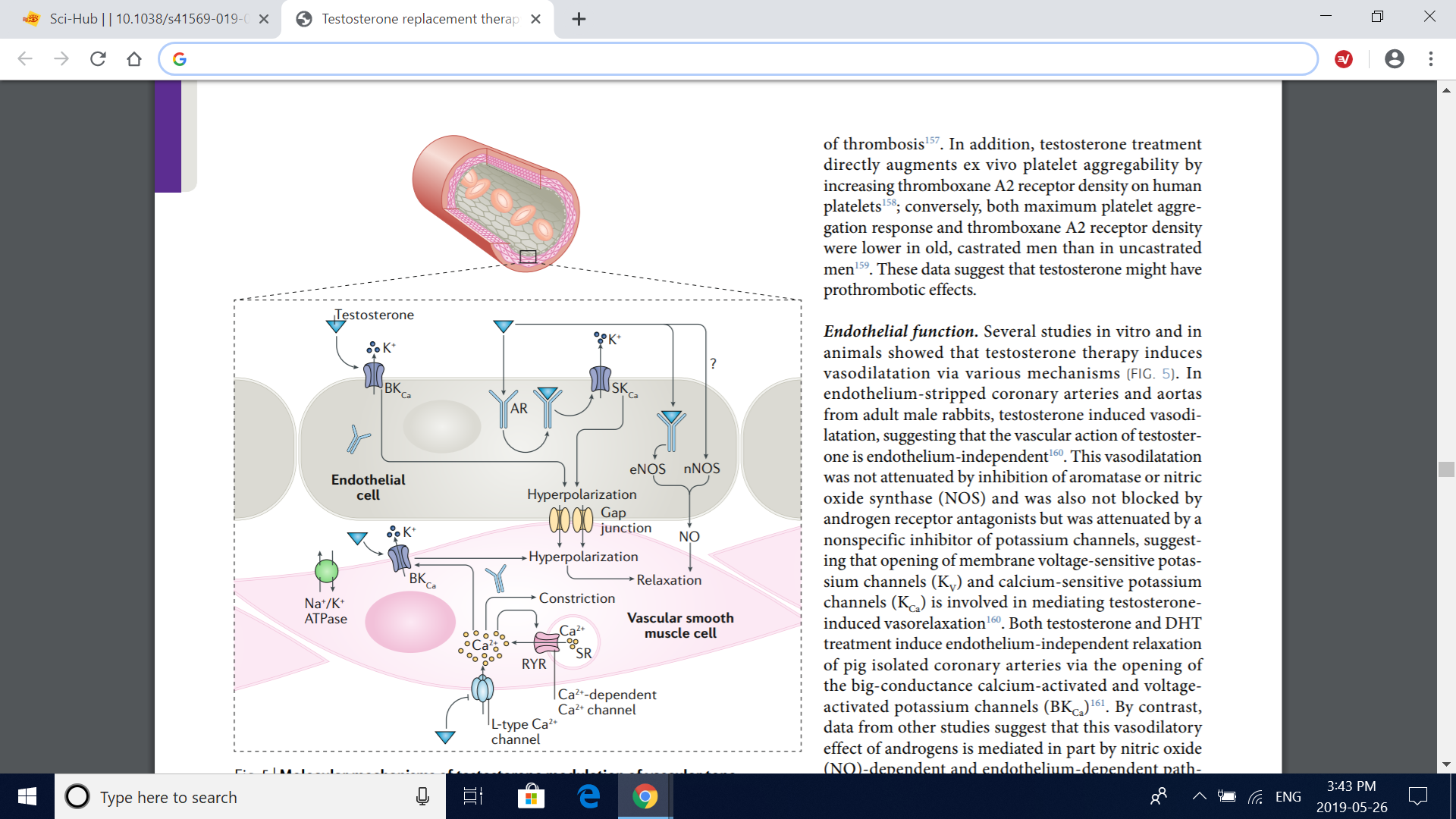
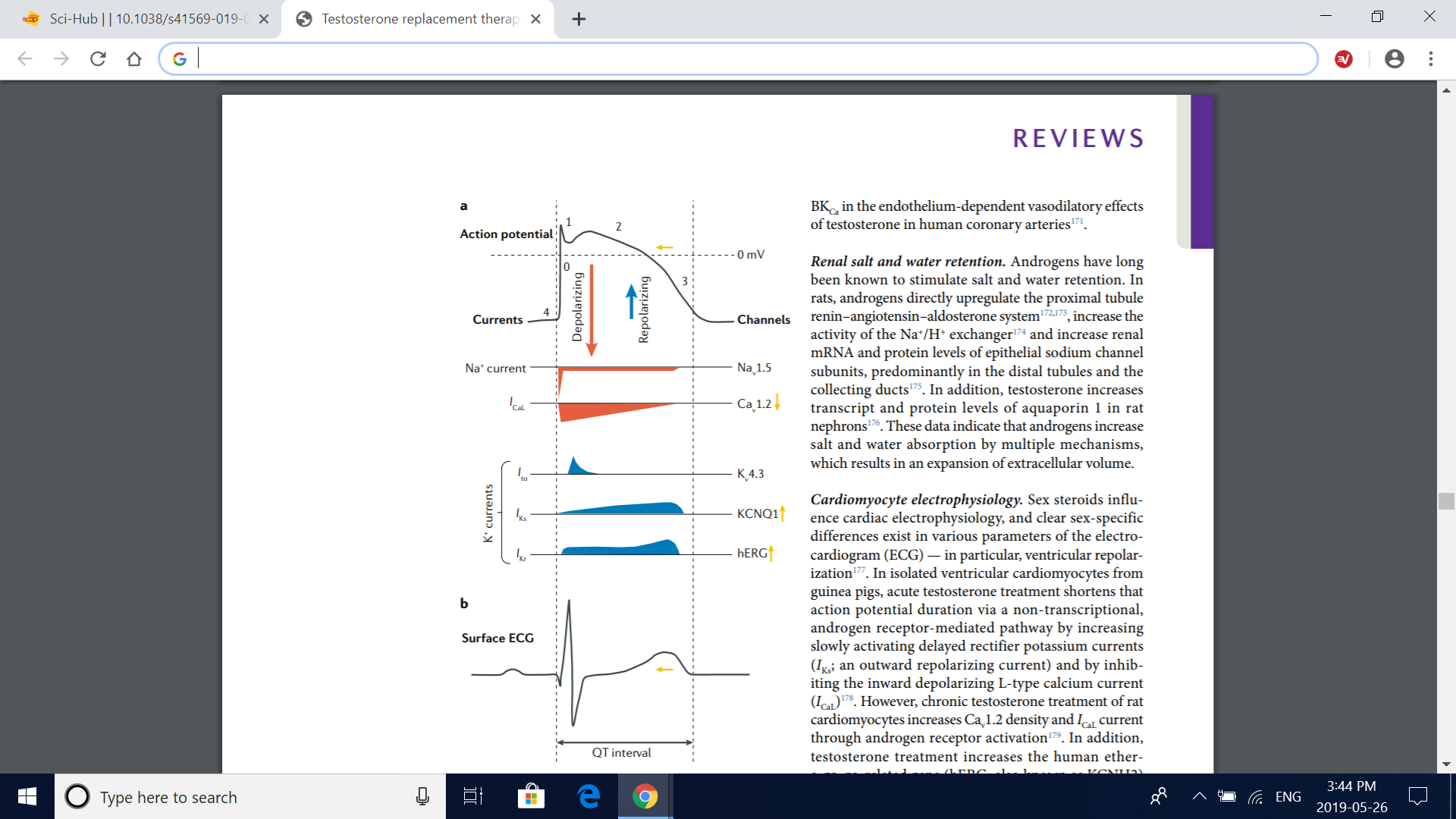
Fig. 6 | Effects of testosterone on cardiac electrophysiology. a | Mechanisms by which testosterone affects the action potential of a ventricular cardiomyocyte. Testosterone has been shown to diminish the L-type calcium current (I CaL) through voltagedependent L-type calcium channel subunit α1C (Cav1.2) voltage-dependent calcium channels, shortening the plateau phase of the action potential (phase 2). In addition, testosterone increases the slowly (I Ks) and rapidly (I Kr) activating delayed rectifier potassium currents — corresponding to the function of voltage-gated potassium channel subfamily Q member 1 (KCNQ1) and human ether-a-go-go-related gene (hERG) potassium channel activities, respectively — accentuating phase 3 (rapid repolarization) of the action potential. Therefore, testosterone seems to shorten the total duration of the action potential by accelerating the recovery of the resting membrane potential (phase 4) without affecting phase 0 (fast depolarization; resulting from the opening of voltage-gated Nav1.5 sodium channels), phase 1 (transient outward potassium current (I to); through Kv4.3 potassium channels) or phase 4. The yellow arrows illustrate the resulting effect of testosterone on each current and on the action potential duration. b | Effect of testosterone on surface electrocardiogram (ECG). The cumulative effect of testosterone on the action potential of ventricular cardiomyocytes results in a shorter ventricular repolarization time, which can be seen in the ECG as a shorter corrected QT interval. Vertical dashed lines illustrate the chronological relationship between the action potential of a ventricular cardiomyocyte (part a) and the surface ECG (part b). Adapted with permission from ref. 268, Elsevier.
The TRAVERSE trial
The TRAVERSE trial 41 is the first randomized, controlled trial that is adequately powered to evaluate the incidence of cardiovascular events with testosterone replacement therapy. Commencing in 2018, the investigators started to randomly assign a planned sample of approximately 6,000 men aged 45–80 years at high risk of cardiovascular disease and with a serum total testosterone level <300ng/dl to receive either testosterone gel or placebo. The planned treatment duration is 5 years, and the primary end point is time to MACE (nonfatal MI, nonfatal stroke or death from cardiovascular causes). Secondary outcomes include time to occurrence of the composite cardiovascular end point (nonfatal MI, nonfatal stroke, death from cardiovascular causes or cardiac revascularization procedures including percutaneous coronary intervention and CABG surgery). The findings of this trial will provide more definitive evidence about the cardiovascular safety of testosterone replacement therapy. Conclusions Testosterone has an important role in cardiovascular physiology
Key points
• Population studies suggest that low serum levels of endogenous testosterone are a risk cardiovascular events, although these studies cannot establish causality or exclude reverse causality, and some of these associations might result from residual confounding.
•Although many retrospective studies show no association, some retrospective studies of prescription databases have shown a higher risk of cardiovascular events in men receiving testosterone, with the risk increasing early after treatment initiation.
•Meta-analyses of randomized, controlled trials of testosterone replacement therapy report conflicting findings, probably because the included trial slacked power or the duration was too short to assess cardiovascular events.
•The TRAVERSE trial, the first trial of testosterone therapy that is adequately powered to assess cardiovascular events, began in 2018, and its findings might take a decade to become available.
•Until the results of the TRAVERSE trial are available, clinicians should individualize testosterone treatment after having an informed discussion with their patients about the risks and benefits of testosterone replacement therapy.
Conclusions
Testosterone has an important role in cardiovascular physiology and metabolic health. Although epidemiological data are conflicting, some retrospective studies support a beneficial effect of testosterone replacement therapy on mortality, whereas other reports suggest that testosterone might increase the risk of serious cardiovascular events, a finding that is also supported by some randomized trials. Meta-analyses also report conflicting results and are limited by the inclusion of low-to-medium quality trials. Furthermore, to date, no published trials of testosterone replacement therapy have been adequately powered to assess cardiovascular events. Therefore, the TRAVERSE trial 41 will go a long way towards enabling an assessment of the cardiovascular safety of testosterone therapy. In the interim, clinicians should guide their patients in making informed decisions by having an open discussion about the current evidence on the cardiovascular safety of testosterone replacement therapy.
Thiago Gagliano-Jucá and Shehzad Basaria
Abstract
Testosterone is the main male sex hormone and is essential for the maintenance of male secondary sexual characteristics and fertility. Androgen deficiency in young men owing to organic disease of the hypothalamus, pituitary gland or testes has been treated with testosterone replacement for decades without reports of increased cardiovascular events. In the past decade, the number of testosterone prescriptions issued for middle-aged or older men with either age related or obesity-related decline in serum testosterone levels has increased exponentially even though these conditions are not approved indications for testosterone therapy. Some retrospective studies and randomized trials have suggested that testosterone replacement therapy increases the risk of cardiovascular disease, which has led the FDA to release a warning statement about the potential cardiovascular risks of testosterone replacement therapy. However, no trials of testosterone replacement therapy published to date were designed or adequately powered to assess cardiovascular events; therefore, the cardiovascular safety of this therapy remains unclear. In this Review, we provide an overview of epidemiological data on the association between serum levels of endogenous testosterone and cardiovascular disease, prescription database studies on the risk of cardiovascular disease in men receiving testosterone therapy, randomized trials and meta-analyses evaluating testosterone replacement therapy and its association with cardiovascular events and mechanistic studies on the effects of testosterone on the cardiovascular system. Our aim is to help clinicians to make informed decisions when considering testosterone replacement therapy in their patients.
Effect of endogenous testosterone level
Epidemiological studies
Summary of epidemiological studies.
In summary, although conflicting results have been obtained in epidemiological studies, most studies suggest that low serum levels of endogenous testosterone are a risk factor for cardiovascular events, cardiovascular mortality and all-cause mortality. However, these data must be interpreted with caution because population studies, no matter how methodologically rigorous, cannot establish causality or exclude reverse causality. Indeed, the researchers in some of these studies suggested that this association is not causal 3,51. Some investigators suggest that testosterone has a beneficial effect on the risk of cardiovascular disease on the basis of studies in men with prostate cancer who received androgen deprivation therapy, which show that low serum testosterone levels are consistently associated with metabolic syndrome, cardiovascular morbidity and mortality 65–69. However, because serum testosterone levels in these men are in the castrate range, which is profoundly lower than the cut-off thresholds in the above-mentioned population studies, these data must also be interpreted with caution.
Table 1 | association between endogenous testosterone levels in serum and cardiovascular events
Table 2 | Studies on the association between endogenous testosterone levels in serum and mortality
Effect of testosterone therapy
Retrospective studies
In summary, these retrospective, prescription database studies provide useful information on the cardiovascular effects of testosterone replacement therapy (Table 3). However, because of the inherent design limitations of retrospective studies, making firm conclusions about the cardiovascular safety of testosterone therapy is not possible.
Table 3 | retrospective studies on testosterone replacement therapy and risk of cardiovascular events and death
Table 3 (cont.) | retrospective studies on testosterone replacement therapy and risk of cardiovascular events and death
Randomized, controlled trials
The Copenhagen Study Group trial.
The TOM trial.
The TEAAM trial.
The TTrials.
Trials on testosterone as a male contraceptive.
Fig. 2 | Effects of testosterone treatment on coronary artery plaques in clinical trials. a | Changes from baseline in the volume of coronary artery plaques in the cardiovascular substudy of the TTrials79 (CV TTrials). The drawings below the graph depict anatomical representations of the plaque subtypes. Compared with placebo, testosterone treatment for 12 months induced a significant increase in fibrous, soft (noncalcified) and total coronary artery plaque volume, as assessed by coronary CT angiography, whereas no significant treatment effect was seen for fibrous-fatty or calcified coronary artery plaque volume. Bars are mean change from baseline to 12 months with testosterone therapy and placebo adjusted for baseline total testosterone level in the serum (≤200ng/dl or >200ng/dl), age (≤75 years or >75 years), trial site, participation in the main TTrials, use or nonuse of antidepressants and use or nonuse of phosphodiesterase type 5 inhibitors. Error bars are 95% CI. Data are derived from ref. 79. *Significant difference (P<0.05) determined by a linear mixed model with all balancing factors and baseline outcome value as covariates and a random effect for participant. b | Changes from baseline in coronary artery calcium scores in the TEAAM trial77. Compared with baseline, no significant difference was observed between treatment groups in mean estimated yearly change in coronary artery calcium scores, as assessed with multidetector-row CT over 3 years of treatment. Estimates are derived from mixed-effects regression models supplemented by multiple imputation of missing records; 95% CIs were not available. Data are obtained from ref. 77.
Fig. 3 | Meta-analyses of clinical trials of testosterone replacement therapy. The graph depicts the pooled odds ratio of composite cardiovascular events from metaanalyses of clinical trials of testosterone replacement therapy, with the exception of the study by Alexander et al.118, which reported myocardial infarction (MI) and stroke as separate outcomes. The definition of composite cardiovascular events varied across studies, but all included MI, angina, coronary revascularization and stroke. In addition, the report by Calof et al.114 included atrial fibrillation, sudden death and other vascular events; Haddad et al.115 included cardiovascular death and claudication; FernándezBalsells et al.116 included death, peripheral vascular events, changes in blood lipid fractions, changes in fasting glucose level, new onset of diabetes mellitus and hypertension; Xu et al.40 included any events reported by the researchers as cardiac disorders, cardiovascular complaints, cardiovascular events and vascular disorders; Albert et al.117 included death, syncope, arrhythmia and hospital admission for congestive heart failure; and Corona et al.119 included cardiovascular-related death and heart failure reported as serious adverse event
Fig. 4 | Cardiovascular targets and effects of testosterone. Studies in vitro, in animals and in humans have examined the effects of testosterone on various organs and processes. Some studies have shown that testosterone modulates vascular tone, increases erythropoiesis (which can induce erythrocytosis in some men) and affects platelet aggregability and cardiomyocyte electrophysiology and contractile activity. In addition, conflicting findings have been reported in studies evaluating the effect of serum levels of endogenous testosterone and testosterone therapy on atherosclerosis progression and inflammation and on metabolic parameters such as insulin sensitivity and lipid homeostasis; these metabolic effects probably involve complex interactions of the liver, skeletal muscle and adipose tissue.
Fig. 5 | Molecular mechanisms of testosterone modulation of vascular tone. Testosterone has effects in both vascular endothelial cells and vascular smooth muscle cells, which together produce changes in vascular tone. Testosterone relaxes the vascular smooth muscle by inhibiting the L-type calcium current through voltagedependent L-type calcium channel subunit α1C (Cav1.2), independently of the vascular endothelium or the androgen receptor (AR). Testosterone also induces vascular smooth muscle relaxation via the opening of big-conductance calcium-activated and voltage-activated potassium channels (BKCa) in vascular smooth muscle cell as well as in endothelial cells. In addition, testosterone induces nitric oxide (NO) synthesis in the endothelial cell by endothelial nitric oxide synthase (eNOS) via an AR-dependent, nontranscriptional mechanism; neuronal nitric oxide synthase (nNOS) in the endothelium might also contribute to the testosterone-induced increase in endothelial NO synthesis. Involvement of small-conductance calcium-activated potassium channels (SKCa) in addition to BKCa in the endothelium-dependent vasodilatory effects of testosterone has also been suggested. RYR, ryanodine receptor; SR, sarcoplasmic reticulum.
Fig. 6 | Effects of testosterone on cardiac electrophysiology. a | Mechanisms by which testosterone affects the action potential of a ventricular cardiomyocyte. Testosterone has been shown to diminish the L-type calcium current (I CaL) through voltagedependent L-type calcium channel subunit α1C (Cav1.2) voltage-dependent calcium channels, shortening the plateau phase of the action potential (phase 2). In addition, testosterone increases the slowly (I Ks) and rapidly (I Kr) activating delayed rectifier potassium currents — corresponding to the function of voltage-gated potassium channel subfamily Q member 1 (KCNQ1) and human ether-a-go-go-related gene (hERG) potassium channel activities, respectively — accentuating phase 3 (rapid repolarization) of the action potential. Therefore, testosterone seems to shorten the total duration of the action potential by accelerating the recovery of the resting membrane potential (phase 4) without affecting phase 0 (fast depolarization; resulting from the opening of voltage-gated Nav1.5 sodium channels), phase 1 (transient outward potassium current (I to); through Kv4.3 potassium channels) or phase 4. The yellow arrows illustrate the resulting effect of testosterone on each current and on the action potential duration. b | Effect of testosterone on surface electrocardiogram (ECG). The cumulative effect of testosterone on the action potential of ventricular cardiomyocytes results in a shorter ventricular repolarization time, which can be seen in the ECG as a shorter corrected QT interval. Vertical dashed lines illustrate the chronological relationship between the action potential of a ventricular cardiomyocyte (part a) and the surface ECG (part b). Adapted with permission from ref. 268, Elsevier.
The TRAVERSE trial
The TRAVERSE trial 41 is the first randomized, controlled trial that is adequately powered to evaluate the incidence of cardiovascular events with testosterone replacement therapy. Commencing in 2018, the investigators started to randomly assign a planned sample of approximately 6,000 men aged 45–80 years at high risk of cardiovascular disease and with a serum total testosterone level <300ng/dl to receive either testosterone gel or placebo. The planned treatment duration is 5 years, and the primary end point is time to MACE (nonfatal MI, nonfatal stroke or death from cardiovascular causes). Secondary outcomes include time to occurrence of the composite cardiovascular end point (nonfatal MI, nonfatal stroke, death from cardiovascular causes or cardiac revascularization procedures including percutaneous coronary intervention and CABG surgery). The findings of this trial will provide more definitive evidence about the cardiovascular safety of testosterone replacement therapy. Conclusions Testosterone has an important role in cardiovascular physiology
Key points
• Population studies suggest that low serum levels of endogenous testosterone are a risk cardiovascular events, although these studies cannot establish causality or exclude reverse causality, and some of these associations might result from residual confounding.
•Although many retrospective studies show no association, some retrospective studies of prescription databases have shown a higher risk of cardiovascular events in men receiving testosterone, with the risk increasing early after treatment initiation.
•Meta-analyses of randomized, controlled trials of testosterone replacement therapy report conflicting findings, probably because the included trial slacked power or the duration was too short to assess cardiovascular events.
•The TRAVERSE trial, the first trial of testosterone therapy that is adequately powered to assess cardiovascular events, began in 2018, and its findings might take a decade to become available.
•Until the results of the TRAVERSE trial are available, clinicians should individualize testosterone treatment after having an informed discussion with their patients about the risks and benefits of testosterone replacement therapy.
Conclusions
Testosterone has an important role in cardiovascular physiology and metabolic health. Although epidemiological data are conflicting, some retrospective studies support a beneficial effect of testosterone replacement therapy on mortality, whereas other reports suggest that testosterone might increase the risk of serious cardiovascular events, a finding that is also supported by some randomized trials. Meta-analyses also report conflicting results and are limited by the inclusion of low-to-medium quality trials. Furthermore, to date, no published trials of testosterone replacement therapy have been adequately powered to assess cardiovascular events. Therefore, the TRAVERSE trial 41 will go a long way towards enabling an assessment of the cardiovascular safety of testosterone therapy. In the interim, clinicians should guide their patients in making informed decisions by having an open discussion about the current evidence on the cardiovascular safety of testosterone replacement therapy.
Attachments
-
[email protected]2 MB · Views: 200
Last edited:

















