madman
Super Moderator
Study Overview
Brief Summary
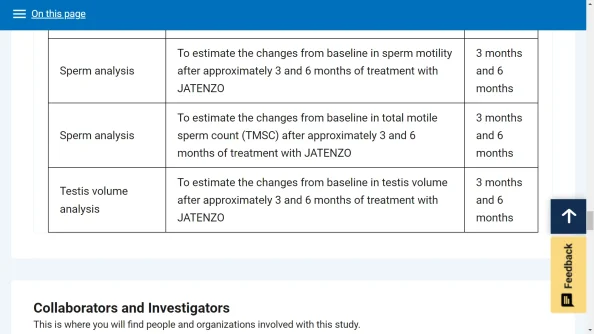
TOL-CLAR-20024 is a Phase 4, multi-center, open-label safety study evaluating the potential effect of JATENZO on adrenal function in hypogonadal men treated with JATENZO for 12 months.
Detailed Description
Following all screening activities and confirmation that the subject has met all eligibility requirements, JATENZO treatment for approximately 1 year will begin. The dose of JATENZO will be titrated using its standard dose-titration algorithms based on serum testosterone levels. The Treatment Period will consist of Visits 1 - 12, with cosyntropin stimulation test completed at Visit 9 (Day 169 ±7) and Visit 12 (Day 365 ±7). At each visit during the Treatment Period, subjects will be evaluated for signs and symptoms of adrenal insufficiency, occurrence of adverse events, and changes in concomitant medications. There will be a safety follow-up visit 2 weeks after completion of Visit 12.