madman
Super Moderator

Revvity Announces FDA Clearance for First Automated Free Testosterone Test
Revvity, Inc. (NYSE: RVTY), today announced that it received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for EUROIMMUN’s automated chem...
www.businesswire.com
* The state-of-the-art assay is processed on the Company’s random-access iSYSTM or i10 TM instruments to deliver quick turnaround times and high-throughput testing with minimal technician training and expertise, while maintaining superior accuracy and reliability.
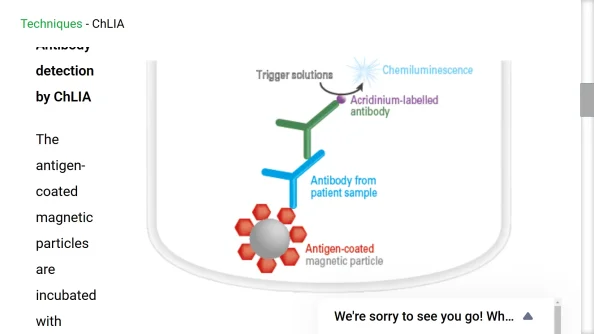
WALTHAM, Mass.--(BUSINESS WIRE)--Revvity, Inc. (NYSE: RVTY), today announced that it received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for EUROIMMUN’s automated chemiluminescence-based immunoassay (ChLIA) test for free testosterone. This innovative test is the first of its kind to receive FDA clearance for direct quantitative measurement of free testosterone levels, marking a significant advancement in diagnostic capabilities for androgen disorders.
Key features of the new test include:
• The only FDA-cleared ChLIA assay for direct quantitative measurement of free testosterone in human serum or plasma.
• Rapid results on EUROIMMUN’s ChLIA platforms with the first result available in just 48 minutes and an estimated throughput of nearly 60 tests per hour.
• Incorporation of monoclonal antibodies to ensure specificity and consistent performance across test batches.
The state-of-the-art assay is processed on the Company’s random-access iSYSTM or i10 TM instruments to deliver quick turnaround times and high-throughput testing with minimal technician training and expertise, while maintaining superior accuracy and reliability. The assay provides direct measurement of free testosterone levels in a single test, enhancing diagnostic capabilities for conditions such as hypogonadism, impotence, polycystic ovarian syndrome (PCOS), and other androgenital syndromes.
"Laboratory customers have been asking for a user-friendly FDA-cleared test, on a random-access platform, for direct measurement of free testosterone," said Jonathan Friend, general manager at Revvity’s EUROIMMUN US. "This clearance reinforces our commitment to expanding the FDA-cleared menu for the EUROIMMUN family of ChLIA automation solutions with assays that serve diverse patient populations across all demographics.”