madman
Super Moderator
Early Experience with Subcutaneous Injection of Non-Proprietary Testosterone as an Alternative Approach for Testosterone Replacement (2022)
KAlter, DRoadman, CAmarasekera, LLevine
Introduction
Testosterone replacement therapy (TRT) has been demonstrated to benefit men with a diagnosis of hypogonadism. We elected to offer a non-proprietary subcutaneous injection (subQ) using a ½ inch 27g needle for TRT as an alternative treatment for hypogonadism.
Objective
To investigate the effect of subQ TRT in hypogonadal men, in order to compare satisfaction, HCT, and PSA levels to other forms of TRT.
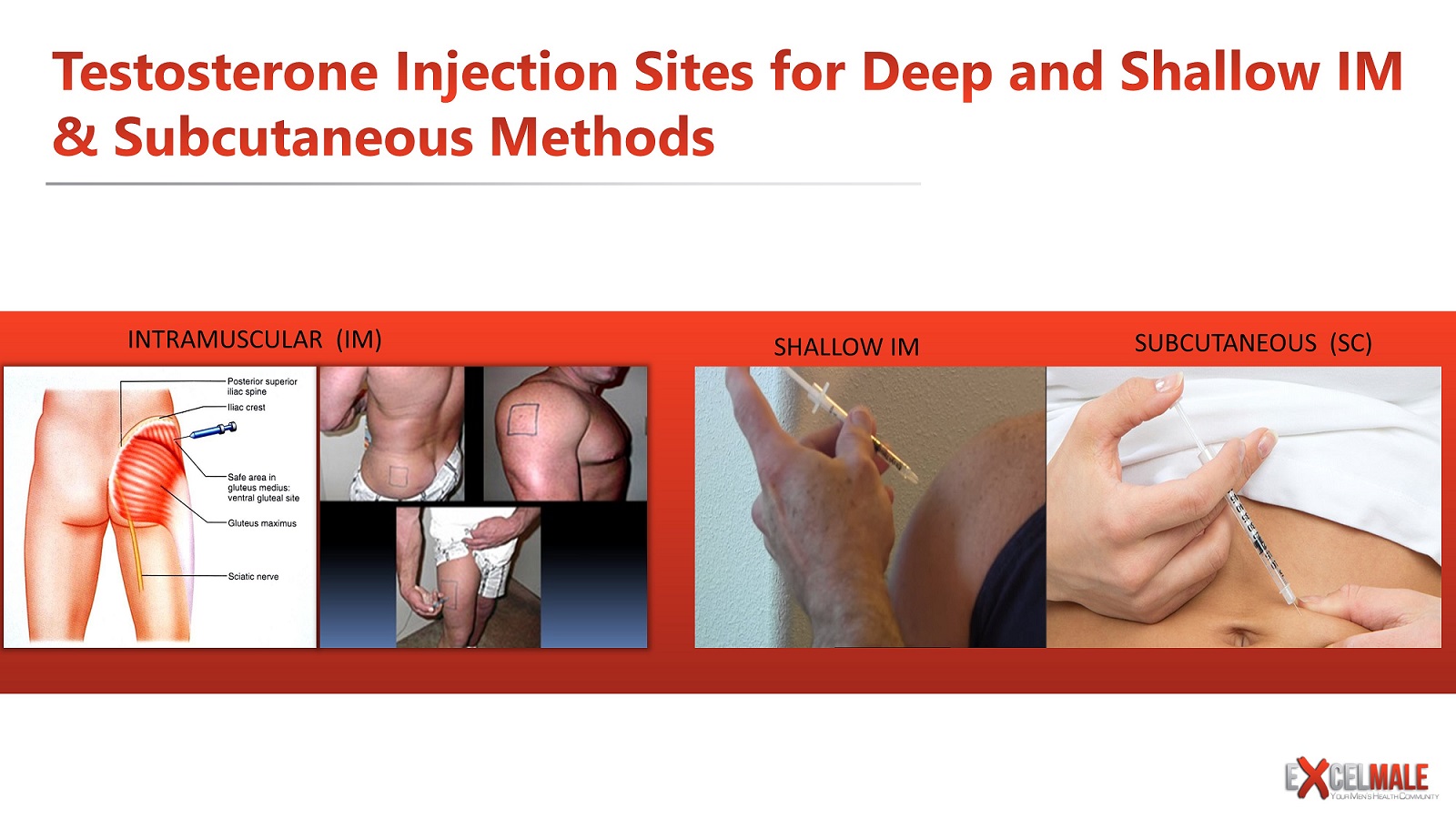
Methods
The study period was defined as the most recent follow-up appointment after beginning subQ TRT with a mean follow-up of 4.2 months (range 1-6 months). Variables were obtained prior to beginning subQ TRT and at the last follow-up visit. Patients either began subQ TRT primarily or switched from another method. Additionally, patients completed a satisfaction survey. Statistical analysis was performed with SPSS 24. Associations between testosterone levels and clinical variables were analyzed by univariate analysis.
Results
32 total patients received subQ TRT. Pre and post-subQ TRT testosterone levels (increased 299.7 +/- 61.5), HCT levels (increased 0.8 +/- 1.0%), PSA levels (increased 0.87 +/- 0.3 ng/mL), AUASS (decreased 3.7 +/- 1.7), and SHIM scores (increased 2.1 +/- 2.4) were recorded (Table 1). 21 patients received other forms of TRT prior to beginning subQ TRT and variables were collected and analyzed (Table 2). Overall, 21 patients completed the follow-up satisfaction survey. 19 (90.5%) were satisfied with subQ TRT and 2 (9.5%) patients attributed their dissatisfaction to greater symptom improvement on previous therapies (Testopel, Jatenzo). 11 (84.6%) patients reported better or same satisfaction on subQ TRT than with prior therapy. Furthermore, 8 (88.9%) patients said subQ TRT was easier to use than prior TRT injection (Intramuscular and Testopel), while 1 (11.1%) patient preferred fewer treatments with Testopel. Lastly, all patients reported subQ TRT as less painful during administration.
Conclusions
SubQ TRT led to higher testosterone levels, positive satisfaction scores, and improved AUASS and SHIM scores at the most recent follow-up. There was also no significant change to HCT or PSA levels. Ongoing monitoring focusing on longer duration of follow-up, erythrocytosis, satisfaction, and side effects are being conducted and will be reported.
KAlter, DRoadman, CAmarasekera, LLevine
Introduction
Testosterone replacement therapy (TRT) has been demonstrated to benefit men with a diagnosis of hypogonadism. We elected to offer a non-proprietary subcutaneous injection (subQ) using a ½ inch 27g needle for TRT as an alternative treatment for hypogonadism.
Objective
To investigate the effect of subQ TRT in hypogonadal men, in order to compare satisfaction, HCT, and PSA levels to other forms of TRT.
Methods
The study period was defined as the most recent follow-up appointment after beginning subQ TRT with a mean follow-up of 4.2 months (range 1-6 months). Variables were obtained prior to beginning subQ TRT and at the last follow-up visit. Patients either began subQ TRT primarily or switched from another method. Additionally, patients completed a satisfaction survey. Statistical analysis was performed with SPSS 24. Associations between testosterone levels and clinical variables were analyzed by univariate analysis.
Results
32 total patients received subQ TRT. Pre and post-subQ TRT testosterone levels (increased 299.7 +/- 61.5), HCT levels (increased 0.8 +/- 1.0%), PSA levels (increased 0.87 +/- 0.3 ng/mL), AUASS (decreased 3.7 +/- 1.7), and SHIM scores (increased 2.1 +/- 2.4) were recorded (Table 1). 21 patients received other forms of TRT prior to beginning subQ TRT and variables were collected and analyzed (Table 2). Overall, 21 patients completed the follow-up satisfaction survey. 19 (90.5%) were satisfied with subQ TRT and 2 (9.5%) patients attributed their dissatisfaction to greater symptom improvement on previous therapies (Testopel, Jatenzo). 11 (84.6%) patients reported better or same satisfaction on subQ TRT than with prior therapy. Furthermore, 8 (88.9%) patients said subQ TRT was easier to use than prior TRT injection (Intramuscular and Testopel), while 1 (11.1%) patient preferred fewer treatments with Testopel. Lastly, all patients reported subQ TRT as less painful during administration.
Conclusions
SubQ TRT led to higher testosterone levels, positive satisfaction scores, and improved AUASS and SHIM scores at the most recent follow-up. There was also no significant change to HCT or PSA levels. Ongoing monitoring focusing on longer duration of follow-up, erythrocytosis, satisfaction, and side effects are being conducted and will be reported.
















