SAN DIEGO — A novel oral testosterone formulation (LPCN 1021, Lipocine), which was shown to be safe and effective at 13 weeks, continues to demonstrate similar results at 52 weeks, according to new data.
The formulation is currently under review at the US Food and Drug Administration (FDA).
Longer-term safety data are especially important because other testosterone pills in the United States have been "rarely" used because of liver toxicity, said Mohit Khera, MD, a urologist at the Baylor College of Medicine in Houston.
Dr Khera presented 52-week results from the phase 3 Study of Androgen Replacement (SOAR) here at the American Urological Association 2016 Annual Meeting.
"There is no liver toxicity because LPCN 1021 is absorbed by the lymphatic system," he told Medscape Medical News. "There's no liver toxicity at all," confirmed Tobias Köhler, MD, a urologist at Southern Illinois University in Springfield, who moderated the press conference at which Dr Khera spoke.
SOAR was a "well-done trial," said Dr Köhler. "The science is very good."
In the multicenter open-label trial of 315 hypogonadal men, 210 men were randomized to twice-daily oral LPCN 1021 at a starting dose of 225 mg and 105 men were randomized to testosterone gel 1.62%.
All men were 18 to 80 years of age and had testosterone levels below 300 ng/dL.
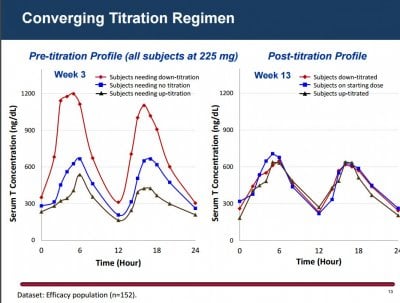
The LPCN 1021 dose could be titrated up if 24-hour average testosterone concentration remained below 300 ng/dL, and could be titrated down if maximum concentration was above 1500 ng/dL.
For this formulation of LPCN 1021, the FDA set average testosterone concentrations from 300 to 1140 ng/dL.
At week 13, the mean 24-hour average testosterone concentration was 446 ng/dL, consistent with nonoral testosterone replacement therapies, as previously reported by Medscape Medical News.
The new data show that those week 13 levels "were reliably maintained through 52 weeks."
Specifically, levels were reliably restored and maintained in the eugonadal range for approximately 87% of hypogonadal men over 52 weeks.
"The efficacy is on par with androgel," Dr Köhler noted. The levels are "excellent."
If the product is approved by the FDA, it will likely be well received by clinicians and patients. "This is the most convenient testosterone formulation we have," he said.
And now, for the first time, what is convenient has been shown to be safe, he added, echoing Dr Khera's comments about the hepatotoxicity of other oral testosterones available in the United States.
Gastrointestinal disorders are an adverse event of concern with oral therapy, said Dr Khera, but there was no significant difference in adverse events between the LPCN 1021 group and the testosterone gel group.
The most common drug-related adverse events over the 52 weeks for LPCN 1021 and testosterone gel were acne (2.9% vs 2.9%), headache (0.5% vs 3.8%), weight increase (2.4% vs 0.0%), hematocrit increase (1.9% vs 0.0%), liver enzyme level increase (1.4 % vs 0.0%), fatigue (0.5% vs 1.9%), and hypertension (0.5% vs 1.9%).
Lipid parameters (cholesterol, low-density lipoprotein, high-density lipoprotein, and triglycerides) were comparable in the two treatment groups at week 52.
Changes in androgenic parameters from baseline to week 52 — such as hematocrit, hemoglobin, platelet, prothrombin, and prostate-specific antigen — were not significantly different between the two groups.
Dr Khera pointed out that LPCN 1021 must be taken with a meal that includes 15 g of fat, which is the amount in a sausage biscuit, half a cup of trail mix, or two pancakes.
Dr Khera reports financial ties to Lipocine, AbbVie, Endo Pharmaceuticals, and Repros.
American Urological Association (AUA) 2016 Annual Meeting: Abstract PD50-09. To be presented May 10, 2016.
The formulation is currently under review at the US Food and Drug Administration (FDA).
Longer-term safety data are especially important because other testosterone pills in the United States have been "rarely" used because of liver toxicity, said Mohit Khera, MD, a urologist at the Baylor College of Medicine in Houston.
Dr Khera presented 52-week results from the phase 3 Study of Androgen Replacement (SOAR) here at the American Urological Association 2016 Annual Meeting.
"There is no liver toxicity because LPCN 1021 is absorbed by the lymphatic system," he told Medscape Medical News. "There's no liver toxicity at all," confirmed Tobias Köhler, MD, a urologist at Southern Illinois University in Springfield, who moderated the press conference at which Dr Khera spoke.
SOAR was a "well-done trial," said Dr Köhler. "The science is very good."
In the multicenter open-label trial of 315 hypogonadal men, 210 men were randomized to twice-daily oral LPCN 1021 at a starting dose of 225 mg and 105 men were randomized to testosterone gel 1.62%.
All men were 18 to 80 years of age and had testosterone levels below 300 ng/dL.
The LPCN 1021 dose could be titrated up if 24-hour average testosterone concentration remained below 300 ng/dL, and could be titrated down if maximum concentration was above 1500 ng/dL.
For this formulation of LPCN 1021, the FDA set average testosterone concentrations from 300 to 1140 ng/dL.
At week 13, the mean 24-hour average testosterone concentration was 446 ng/dL, consistent with nonoral testosterone replacement therapies, as previously reported by Medscape Medical News.
The new data show that those week 13 levels "were reliably maintained through 52 weeks."
Specifically, levels were reliably restored and maintained in the eugonadal range for approximately 87% of hypogonadal men over 52 weeks.
"The efficacy is on par with androgel," Dr Köhler noted. The levels are "excellent."
If the product is approved by the FDA, it will likely be well received by clinicians and patients. "This is the most convenient testosterone formulation we have," he said.
And now, for the first time, what is convenient has been shown to be safe, he added, echoing Dr Khera's comments about the hepatotoxicity of other oral testosterones available in the United States.
Gastrointestinal disorders are an adverse event of concern with oral therapy, said Dr Khera, but there was no significant difference in adverse events between the LPCN 1021 group and the testosterone gel group.
The most common drug-related adverse events over the 52 weeks for LPCN 1021 and testosterone gel were acne (2.9% vs 2.9%), headache (0.5% vs 3.8%), weight increase (2.4% vs 0.0%), hematocrit increase (1.9% vs 0.0%), liver enzyme level increase (1.4 % vs 0.0%), fatigue (0.5% vs 1.9%), and hypertension (0.5% vs 1.9%).
Lipid parameters (cholesterol, low-density lipoprotein, high-density lipoprotein, and triglycerides) were comparable in the two treatment groups at week 52.
Changes in androgenic parameters from baseline to week 52 — such as hematocrit, hemoglobin, platelet, prothrombin, and prostate-specific antigen — were not significantly different between the two groups.
Dr Khera pointed out that LPCN 1021 must be taken with a meal that includes 15 g of fat, which is the amount in a sausage biscuit, half a cup of trail mix, or two pancakes.
Dr Khera reports financial ties to Lipocine, AbbVie, Endo Pharmaceuticals, and Repros.
American Urological Association (AUA) 2016 Annual Meeting: Abstract PD50-09. To be presented May 10, 2016.