Nelson Vergel
Founder, ExcelMale.com
Recently presented at a Urology conference Nov 2018 in Miami.
Title: Nandrolone Decanoate Improves Joint Pain in Hypogonadal Men Within 8 Weeks: A Novel Prospective Pilot Study
Authors: Alexander J. Tatem, Jonathan A. Beilan, Jason R. Kovac, Jabez Gondokusumo, Nannan Thirumavalavan, Larry I. Lipshultz
Introduction and Objective
5-20% of adult men suffer from hypogonadism (HG). Comorbidities linked with HG, such as diabetes and obesity, are often associated with significant and debilitating joint pain (JP). Nandrolone decanoate (ND) is an FDA approved testosterone derivative for treatment of anemia and muscle-wasting syndrome that has anecdotally been linked to reduced JP. Here we quantify this effect prospectively in a novel pilot study.
Methods
Hypogonadal men taking injectable testosterone therapy (TTh) presenting to a single andrology clinic between July 2018 and October 2018 were evaluated for the presence of JP. Men who reported significant JP and denied prior ND usage were invited to take part in the study.
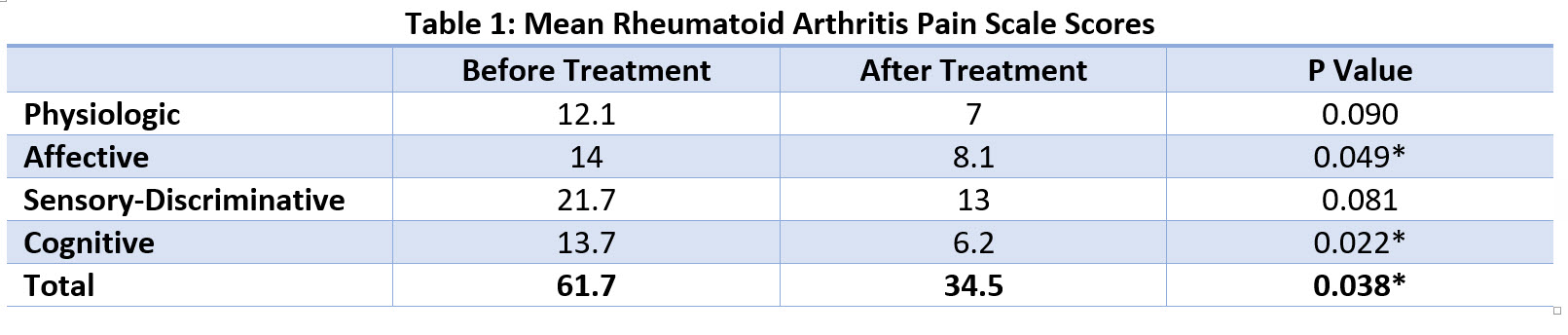
The Rheumatoid Arthritis Pain Scale (RAPS) is a validated questionnaire initially developed to assess/characterize pain levels in adults with rheumatoid arthritis. It contains 24 statements about JP that patients assign a value ranging from 0 (never) to 6 (always). Pain scores are totaled with higher scores representing worse pain and can then be divided into physiologic, affective, sensory-discriminative, and cognitive components.
Men were asked to complete the RAPS questionnaire prior to starting ND. Patient-specific characteristics were recorded, including pain location and pain medication use/dosages. Men subsequently started injectable ND at half the dosage of their current testosterone regimen with all other medications kept constant. After 8 weeks, they filled out the survey again.
Results
32 eligible patients completed the initial survey and 11 men (34.4%) responded to follow-up requests at the time of this review. Mean duration of therapy was 54 days. All patients reported marked improvements in JP with 4 (36.4%) reporting a decreased need for pain medication. Patients’ total pain scores decreased from an average of 61.7 to 34.5 (p=0.038). Significant improvements in each sub-category were noted (Table 1). No adverse events were reported.
Conclusions
ND is a promising new adjunctive therapy for hypogonadal men with JP. It reduced pain scores by an average of 44% and decreased pain medication requirements in 36.4% of patients. Reducing pain medication needs is paramount in today’s opioid crisis climate. Further studies are required to better characterize ND’s effects across a larger study population.
All About Nandrolone
Source of Funding: This work is supported in part by NIH grant K12 DK0083014, the Multidisciplinary K12 Urologic Research (KURe) Career Development Program (NT is a K12 Scholar).
Title: Nandrolone Decanoate Improves Joint Pain in Hypogonadal Men Within 8 Weeks: A Novel Prospective Pilot Study
Authors: Alexander J. Tatem, Jonathan A. Beilan, Jason R. Kovac, Jabez Gondokusumo, Nannan Thirumavalavan, Larry I. Lipshultz
Introduction and Objective
5-20% of adult men suffer from hypogonadism (HG). Comorbidities linked with HG, such as diabetes and obesity, are often associated with significant and debilitating joint pain (JP). Nandrolone decanoate (ND) is an FDA approved testosterone derivative for treatment of anemia and muscle-wasting syndrome that has anecdotally been linked to reduced JP. Here we quantify this effect prospectively in a novel pilot study.
Methods
Hypogonadal men taking injectable testosterone therapy (TTh) presenting to a single andrology clinic between July 2018 and October 2018 were evaluated for the presence of JP. Men who reported significant JP and denied prior ND usage were invited to take part in the study.
The Rheumatoid Arthritis Pain Scale (RAPS) is a validated questionnaire initially developed to assess/characterize pain levels in adults with rheumatoid arthritis. It contains 24 statements about JP that patients assign a value ranging from 0 (never) to 6 (always). Pain scores are totaled with higher scores representing worse pain and can then be divided into physiologic, affective, sensory-discriminative, and cognitive components.
Men were asked to complete the RAPS questionnaire prior to starting ND. Patient-specific characteristics were recorded, including pain location and pain medication use/dosages. Men subsequently started injectable ND at half the dosage of their current testosterone regimen with all other medications kept constant. After 8 weeks, they filled out the survey again.
Results
32 eligible patients completed the initial survey and 11 men (34.4%) responded to follow-up requests at the time of this review. Mean duration of therapy was 54 days. All patients reported marked improvements in JP with 4 (36.4%) reporting a decreased need for pain medication. Patients’ total pain scores decreased from an average of 61.7 to 34.5 (p=0.038). Significant improvements in each sub-category were noted (Table 1). No adverse events were reported.
Conclusions
ND is a promising new adjunctive therapy for hypogonadal men with JP. It reduced pain scores by an average of 44% and decreased pain medication requirements in 36.4% of patients. Reducing pain medication needs is paramount in today’s opioid crisis climate. Further studies are required to better characterize ND’s effects across a larger study population.
All About Nandrolone
Source of Funding: This work is supported in part by NIH grant K12 DK0083014, the Multidisciplinary K12 Urologic Research (KURe) Career Development Program (NT is a K12 Scholar).
Last edited:
















