Michael Scally
Member
It Takes a While for Your Body to Start your Own Testosterone and Sperm Production
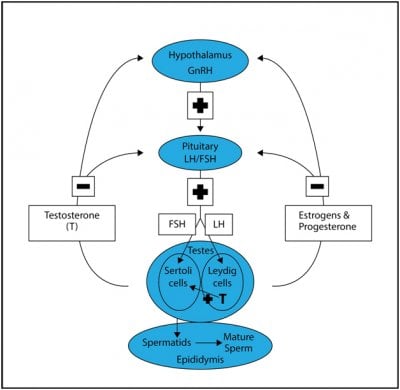
Some men may want to stop testosterone replacement therapy (TRT) or anabolic androgenic steroids (AAS) due to side effects or the wish to improve fertility. We know that stopping TRT or AAS suddenly can cause declines in quality of life amnd libido as they become hypogonadal due to the shut down of the HPT Axis for a few weeks or month.
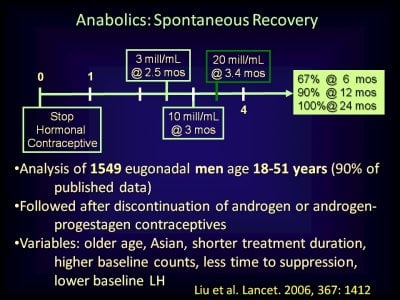
In clinical trials of testosterone-based male hormone regimens, serum gonadotropin and testosterone concentrations typically normalize within 1 to 3 months and sperm concentrations typically normalize within 12 to 16 months after cessation of exogenous testosterone. These male hormone contraceptive regimens have used physiologic to modestly supraphysiologic dosages of testosterone for <2 years. A 2017 meta-analysis of the effects of much higher dosages of potent anabolic androgenic steroid (AASs) on the reproductive system of users found only 33 studies (1766 users and 2113 nonuser controls) that met criteria for inclusion; one-half of the subjects were in one cohort study) . Three studies were randomized, controlled trials, and six studies (no randomized, controlled trials) had data on women. There was substantial heterogeneity of the studies and the risk of bias in the studies was unclear. There were seven studies with hormone data (testosterone and/or gonadotropin concentrations) after immediate cessation of AASs and during recovery, and there were three studies (n = 17 AAS users) with hormone data before initiation of AAS use and during recovery from cessation of AAS use. The studies included in this meta-analysis generally had an AAS exposure ≤1 year. Based on limited data, it appears that serum gonadotropins recover to normal concentrations within 3 to 6 months, followed by recovery of serum testosterone to baseline within 6 months in most but not all men who use AASs ≤1 year. Case series have shown that many men who use high-dosage AASs for >1 year will have symptoms suggestive of hypogonadism and serum testosterone concentrations below normal for years after cessation of AAS use.
The Use of Clomiphene (Clomid) to Help Men Who are Stopping Testosterone or AASs
Clomiphene is a selective estrogen receptor modulator that increases serum gonadotropins and testosterone concentrations within 2 to 4 weeks in normal men and in men with intact pituitary function and low or normal serum gonadotropin but low serum testosterone concentrations . One 3-month randomized prospective study and three longer-term cohort studies (mean time of therapy, 1.5 to 2 years) reported no substantial adverse events at dosages of up 100 mg every other day in younger men (<50 years old) with baseline low testosterone concentrations. There were no important adverse events in these studies, but safety data did not appear to be systematically reported. In women, selective estrogen receptor modulators (e.g., clomiphene) increase the risk of venous thrombosis. There are case reports of clomiphene-induced venous thrombosis in men. Anecdotal reports suggest that some male chronic AAS users may have recovery of their hypothalamic-pituitary-gonadal axis after clomiphene therapy for AAS withdrawal.
The benefits of normalization of serum gonadotropins and testosterone followed by potentially improved spermatogenesis 3 to 6 months later might be worth the risk of uncertain safety for some men with AAS use >1 year who are willing to quit using AASs and who are attempting to conceive a child with a female partner as soon as possible. A 6- to 12-month trial of clomiphene at a dosage sufficient to increase serum testosterone to the upper half of the normal range is reasonable in these men. Initiate oral clomiphene 25 mg every other day and increase the dosage by 12.5 to 25 mg every other day (up to 100 mg every other day) based on monthly serum testosterone concentrations. After 3 to 4 months of maintenance of serum testosterone concentrations within the normal range, the dosage of clomiphene is reduced by 12.5 mg every other day at monthly intervals. The clinician should inform the patient that the safety and effectiveness of the approach has not been proven and that clomiphene might increase the risk of venous thrombosis. The clinician must document the conversation in the medical record before prescribing clomiphene.
How About hCG?
Similarly, hCG therapy might be useful in the same group of men. Subcutaneous hCG therapy stimulates testosterone production and spermatogenesis in most men with hypogonadotropic hypogonadism with testes that are ≥6 mL. Administration of hCG suppresses endogenous production of LH and FSH; therefore, the gonadal axis will remain suppressed after discontinuation of hCG. The usual dosage of hCG is 1000 to 2000 IU subcutaneously 2 to 3 times weekly. The dosage is adjusted until serum total testosterone is in the normal range and continued at least until conception. There is more clinical experience and published data on the safety and effectiveness of hCG therapy than clomiphene therapy in men, but there are no data on the comparative effectiveness of these drugs. If a man with a history of long-term AAS use is treated with either hCG or clomiphene fails to have increased serum testosterone concentrations after 3 months of treatment, the clinician should consider the possibility of underlying classic causes of hypogonadism such as Klinefelter syndrome or pituitary tumor.
 www.ncbi.nlm.nih.gov
www.ncbi.nlm.nih.gov
_________________________________________________________
In this sample of 59/4,400 men using TRT presenting with infertility, close to 2/3 recovered spermatogenesis within 6 months of T discontinuation.
Samplaski MK, Loai Y, Wong K, Lo KC, Grober ED, Jarvi KA. Testosterone use in the male infertility population: prescribing patterns and effects on semen and hormonal parameters. Fertil Steril. Testosterone use in the male infertility population: prescribing patterns and effects on semen and hormonal parameters
OBJECTIVE: To analyze how frequently and why men presenting with infertility take testosterone (T) and if negative effects of T on semen parameters are reversed following cessation.
DESIGN: Analysis of a prospectively collected database.
SETTING: Male Infertility clinic.
PATIENT(S): Men presenting for fertility evaluation from 2008 to 2012.
INTERVENTION(S): None.
MAIN OUTCOME MEASURE(S): The frequency and reason for T use in the infertile male population, and semen and hormonal parameters while on T and following discontinuation.
RESULT(S): A total of 59/4,400 men (1.3%) reported taking T. T was prescribed by a variety of physicians, including endocrinologists (24%), general practitioners (17%), urologists (15%), gynecologists (5%), and reproductive endocrinologists (3%). Only one of the men admitted that he had obtained T from an illicit source. More than 82% of men were prescribed T for the treatment of hypogonadism, but surprisingly, 12% (7/59) were prescribed T to treat their infertility. While on T, 88.4% of men were azoospermic, but by 6 months after T cessation, 65% of the men without other known causes for azoospermia recovered spermatogenesis.
CONCLUSION(S): In Canada, T was not commonly used by men presenting for fertility investigation (1.3%). Close to 2/3 of infertile men using T recovered spermatogenesis within 6 months of T discontinuation.
Some men may want to stop testosterone replacement therapy (TRT) or anabolic androgenic steroids (AAS) due to side effects or the wish to improve fertility. We know that stopping TRT or AAS suddenly can cause declines in quality of life amnd libido as they become hypogonadal due to the shut down of the HPT Axis for a few weeks or month.
In clinical trials of testosterone-based male hormone regimens, serum gonadotropin and testosterone concentrations typically normalize within 1 to 3 months and sperm concentrations typically normalize within 12 to 16 months after cessation of exogenous testosterone. These male hormone contraceptive regimens have used physiologic to modestly supraphysiologic dosages of testosterone for <2 years. A 2017 meta-analysis of the effects of much higher dosages of potent anabolic androgenic steroid (AASs) on the reproductive system of users found only 33 studies (1766 users and 2113 nonuser controls) that met criteria for inclusion; one-half of the subjects were in one cohort study) . Three studies were randomized, controlled trials, and six studies (no randomized, controlled trials) had data on women. There was substantial heterogeneity of the studies and the risk of bias in the studies was unclear. There were seven studies with hormone data (testosterone and/or gonadotropin concentrations) after immediate cessation of AASs and during recovery, and there were three studies (n = 17 AAS users) with hormone data before initiation of AAS use and during recovery from cessation of AAS use. The studies included in this meta-analysis generally had an AAS exposure ≤1 year. Based on limited data, it appears that serum gonadotropins recover to normal concentrations within 3 to 6 months, followed by recovery of serum testosterone to baseline within 6 months in most but not all men who use AASs ≤1 year. Case series have shown that many men who use high-dosage AASs for >1 year will have symptoms suggestive of hypogonadism and serum testosterone concentrations below normal for years after cessation of AAS use.
The Use of Clomiphene (Clomid) to Help Men Who are Stopping Testosterone or AASs
Clomiphene is a selective estrogen receptor modulator that increases serum gonadotropins and testosterone concentrations within 2 to 4 weeks in normal men and in men with intact pituitary function and low or normal serum gonadotropin but low serum testosterone concentrations . One 3-month randomized prospective study and three longer-term cohort studies (mean time of therapy, 1.5 to 2 years) reported no substantial adverse events at dosages of up 100 mg every other day in younger men (<50 years old) with baseline low testosterone concentrations. There were no important adverse events in these studies, but safety data did not appear to be systematically reported. In women, selective estrogen receptor modulators (e.g., clomiphene) increase the risk of venous thrombosis. There are case reports of clomiphene-induced venous thrombosis in men. Anecdotal reports suggest that some male chronic AAS users may have recovery of their hypothalamic-pituitary-gonadal axis after clomiphene therapy for AAS withdrawal.
The benefits of normalization of serum gonadotropins and testosterone followed by potentially improved spermatogenesis 3 to 6 months later might be worth the risk of uncertain safety for some men with AAS use >1 year who are willing to quit using AASs and who are attempting to conceive a child with a female partner as soon as possible. A 6- to 12-month trial of clomiphene at a dosage sufficient to increase serum testosterone to the upper half of the normal range is reasonable in these men. Initiate oral clomiphene 25 mg every other day and increase the dosage by 12.5 to 25 mg every other day (up to 100 mg every other day) based on monthly serum testosterone concentrations. After 3 to 4 months of maintenance of serum testosterone concentrations within the normal range, the dosage of clomiphene is reduced by 12.5 mg every other day at monthly intervals. The clinician should inform the patient that the safety and effectiveness of the approach has not been proven and that clomiphene might increase the risk of venous thrombosis. The clinician must document the conversation in the medical record before prescribing clomiphene.
How About hCG?
Similarly, hCG therapy might be useful in the same group of men. Subcutaneous hCG therapy stimulates testosterone production and spermatogenesis in most men with hypogonadotropic hypogonadism with testes that are ≥6 mL. Administration of hCG suppresses endogenous production of LH and FSH; therefore, the gonadal axis will remain suppressed after discontinuation of hCG. The usual dosage of hCG is 1000 to 2000 IU subcutaneously 2 to 3 times weekly. The dosage is adjusted until serum total testosterone is in the normal range and continued at least until conception. There is more clinical experience and published data on the safety and effectiveness of hCG therapy than clomiphene therapy in men, but there are no data on the comparative effectiveness of these drugs. If a man with a history of long-term AAS use is treated with either hCG or clomiphene fails to have increased serum testosterone concentrations after 3 months of treatment, the clinician should consider the possibility of underlying classic causes of hypogonadism such as Klinefelter syndrome or pituitary tumor.
Diagnosis and Management of Anabolic Androgenic Steroid Use - PMC
The lifetime prevalence of anabolic androgenic steroid (AAS) use is estimated at 1% to 5% worldwide. AAS use occurs primarily male elite athletes and men who want a muscular appearance. The evidence for effective, safe management of AAS cessation ...
_________________________________________________________
In this sample of 59/4,400 men using TRT presenting with infertility, close to 2/3 recovered spermatogenesis within 6 months of T discontinuation.
Samplaski MK, Loai Y, Wong K, Lo KC, Grober ED, Jarvi KA. Testosterone use in the male infertility population: prescribing patterns and effects on semen and hormonal parameters. Fertil Steril. Testosterone use in the male infertility population: prescribing patterns and effects on semen and hormonal parameters
OBJECTIVE: To analyze how frequently and why men presenting with infertility take testosterone (T) and if negative effects of T on semen parameters are reversed following cessation.
DESIGN: Analysis of a prospectively collected database.
SETTING: Male Infertility clinic.
PATIENT(S): Men presenting for fertility evaluation from 2008 to 2012.
INTERVENTION(S): None.
MAIN OUTCOME MEASURE(S): The frequency and reason for T use in the infertile male population, and semen and hormonal parameters while on T and following discontinuation.
RESULT(S): A total of 59/4,400 men (1.3%) reported taking T. T was prescribed by a variety of physicians, including endocrinologists (24%), general practitioners (17%), urologists (15%), gynecologists (5%), and reproductive endocrinologists (3%). Only one of the men admitted that he had obtained T from an illicit source. More than 82% of men were prescribed T for the treatment of hypogonadism, but surprisingly, 12% (7/59) were prescribed T to treat their infertility. While on T, 88.4% of men were azoospermic, but by 6 months after T cessation, 65% of the men without other known causes for azoospermia recovered spermatogenesis.
CONCLUSION(S): In Canada, T was not commonly used by men presenting for fertility investigation (1.3%). Close to 2/3 of infertile men using T recovered spermatogenesis within 6 months of T discontinuation.