madman
Super Moderator
Sleep, testosterone and cortisol balance, and aging men (2022)
Peter Y. Liu, · Radha T. Reddy
Abstract
Sleep serves important biological functions and influences health and longevity through endocrine and metabolic-related systems. Sleep debt, circadian misalignment, and sleep disruption from obstructive sleep apnea are widespread in modern society and accumulate with life because recovery sleep is not completely restorative. Accumulated disordered sleep throughout life impacts the aging process and the development of age-related diseases. When epidemiological and interventional studies are considered collectively, sleep loss and lower sleep duration are associated with lower morning, afternoon, and 24-h testosterone; as well as higher afternoon, but not morning or 24-h cortisol. These reciprocal changes imbalance anabolic catabolic signaling because testosterone and cortisol are respectively the main anabolics and catabolic signals in man. Fixing testosterone-cortisol balance by means of a novel dual-hormone clamp mitigates the induction of insulin resistance by sleep restriction and provided the first proof of concept that the metabolic harm from sleep loss can be ameliorated by approaches that do not require sleeping more. Obstructive sleep apnea is associated with lower testosterone, even after controlling for age and obesity whereas the conclusion that continuous positive airway pressure therapy has no effect on testosterone is premature because available studies are underpowered and better-quality studies suggest otherwise. High-dose testosterone therapy induces OSA, but more physiological dosing may not, and this effect may be transient or may dissipate with longer-term therapy. Studies investigating the origin of the diurnal testosterone rhythm, the effect of circadian misalignment on testosterone-cortisol balance, and methods to mitigate metabolic harm, are required.
1 Introduction
Sleep restores neurobehavioral performance [1], improves immune function [2], and conserves whole-body energy expenditure through metabolic processes that also restore brain energy stores and are important for neuronal plasticity and connectivity [3]. These sleep-related benefits on metabolism, immunity, and cognition are essential for healthy aging. Conversely, the accumulation of sleep debt across the lifespan negatively impacts metabolic conservation, neurobehavioral performance, immunity, and autoimmunity – impacts that may be responsible for the development of diseases that accrue with age.
Sleep debt accumulates throughout life from repetitive episodes of insufficient sleep (not sleeping for an adequate amount of time), misaligned sleep (as occurs with jetlag or night shiftwork), and disrupted sleep (from obstructive sleep apnea, nocturia associated with aging, or sporadic environmental noise) [4]. This accumulation occurs because intermittent recovery or “catch-up” sleep does not seem to completely reverse the adverse effects of sleep debt on multiple physiological processes including psychomotor performance [1, 5], metabolism [6, 7], blood pressure regulation [8] and immune/adrenal response [9]. Furthermore, the accumulation of sleep debt is widespread in modern society: approximately one in three sleep insufficiently (<7 h per night) [10], up to 20% are shift workers with work schedules that misalign sleep [11], and 10% of men (3% of women) aged 30–49 years and 17% of men (9% of women) aged 50–70 years have at least moderate obstructive sleep apnea [12]. Insufficient, misaligned, and disrupted sleep is therefore likely to have major repercussions on aging and the development of age-related diseases because catch-up sleep is ineffectual and abnormal sleep is widespread in our modern society. An important consideration in determining accumulated sleep debt is that sleep need itself decrease with older age. Another consideration is that the COVID-19 pandemic increased the global prevalence of sleep disturbances, particularly in younger individuals infected with the disease, but whether this trend will persist is not yet known [13]
Sleep may influence health and longevity through endocrine and metabolic systems [14]. This is because endocrine networks evolved to regulate whole-body metabolism, including catabolism and anabolism, in a diurnally appropriate manner, and to simultaneously allow dynamic responses to external environmental insults and internal stress through the ultradian (also known as pulsatile) nature of hormone secretion [15, 16]. An important concept here is that the circadian pattern in hormones allows for anticipation of regular events that occur in a 24 h day (for example to regulate whole-body metabolism), whereas the pulsatile nature of hormone release results in large changes in hormone concentrations in a short period of time which allows for a more rapid and biologically efficient (as opposed to a sustained non-pulsatile release) response to unpredictable events. In this fashion, specific endocrine networks can coordinate growth, puberty, and reproduction in an age- and environment-appropriate manner.
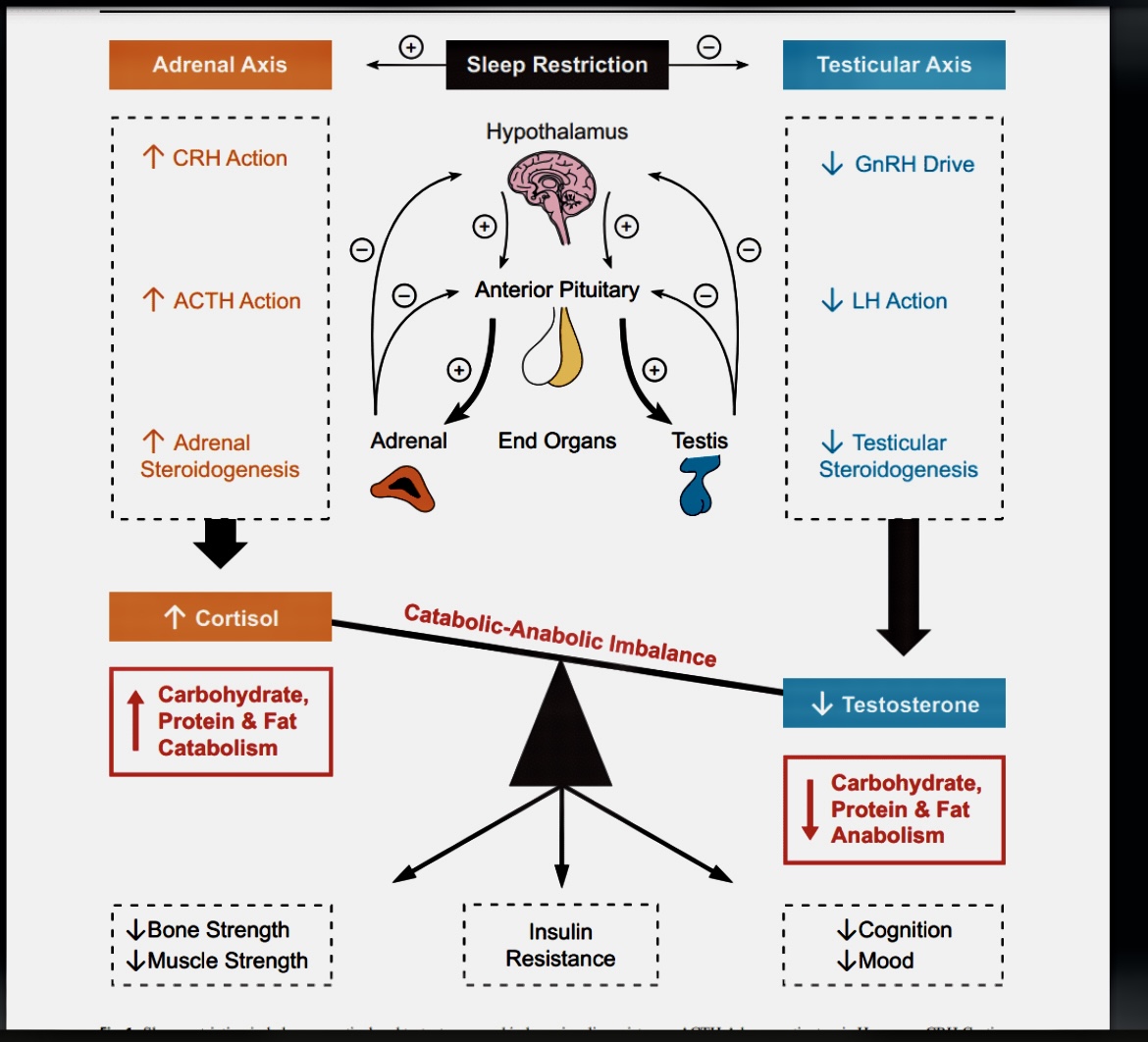
*This review will primarily examine the effect of sleep disturbances on testosterone in the context of male gonadal aging. However, to understand the full impact of restricted sleep on testosterone, it is necessary to determine the net effects on whole body metabolism and metabolic illnesses in conjunction with cortisol: see Fig. 1. This is because the hypothalamic-pituitary testicular and hypothalamic-pituitary-adrenal axes respectively control testosterone, the major anabolic hormone in men (but not women) and cortisol, a key catabolic signal, are intertwined in their regulation and sleep restriction imbalances both their signaling [4]. Obstructive sleep apnea (OSA) and its interaction with testosterone are of particular clinical relevance because sleep architecture is disrupted and sleep duration is reduced. The review will therefore conclude with a discussion of OSA and testosterone in the context of aging.
2 Sleep, sleep architecture, and aging
3 The inter‑relationships between sleep duration, testosterone, and aging
3.1 Testosterone and aging
3.2 Sleep duration, testosterone, and aging
4 The inter‑relationships between sleep duration, cortisol, and aging
4.1 Cortisol and aging
4.2 Sleep duration, cortisol, and aging
5 Testosterone and cortisol imbalance from sleep restriction leads to metabolic harm
6 Effect of circadian misalignment on testosterone and cortisol
7 Obstructive sleep apnea
7.1 OSA, obesity, and testosterone in aging men
7.2 Testosterone effects on OSA
8 Summary and conclusions
Sleep is highly organized, serves important biological functions, and influences health and longevity through endocrine and metabolic-regulated systems. Accumulated sleep debt is widespread in modern society and when accumulated throughout life likely impacts the aging process, and the development of age-related diseases.
Sleep loss and lower sleep duration are associated with lower morning, afternoon, and 24-h testosterone, whereas they are associated with higher late afternoon and early evening, but not morning or 24-h cortisol. These reciprocal changes in testosterone and cortisol with sleep loss imbalances catabolic-anabolic signaling and is an important, but not exclusive, the mechanism by which sleep loss induces insulin resistance. By fixing testosterone-cortisol balance to prevent the induction of insulin resistance by sleep restriction, we provided the first proof of concept that the metabolic harm that occurs with sleep loss can potentially be mitigated by therapeutic approaches that do not require sleeping more. This approach is likely to be relevant also to older men since the changes in testosterone-cortisol balance that occur in young men have recently been shown to also occur in older men.
Epidemiological studies when considered in unison show that OSA is associated with lower testosterone levels, independently of confounders such as age and obesity. Circumstantial evidence would favor the possibility that more severe OSA, due to greater hypoxemia, lowers testosterone although the opposite is plausible. It would be premature to conclude that CPAP therapy has no effect on testosterone in men with OSA since the available studies are underpowered, and the higher quality studies suggest otherwise. In contrast, the larger number of studies available, particularly higher quality studies, has allowed the conclusion to be made that CPAP decreases cortisol. High-dose testosterone therapy induces OSA, but more physiological dosing may not; this effect may be transient or dissipate with longer-term therapy
Important limitations are that only one interventional study has examined the effect of restricting sleep on testosterone and cortisol in a cohort of older men; few studies have been designed to examine changes in relevant testosterone and cortisol pulse characteristics with age and/or in response to sleep manipulation; and longitudinal epidemiological studies examining the age-related changes in sleep architecture, the impact of changes in sleep on testosterone and cortisol, are rudimentary.
Nevertheless, the available data are highly suggestive that restricted sleep, circadian misalignment, and disrupted sleep from OSA are relevant to age and age-related diseases through alterations in testosterone and cortisol signaling. Therefore, society should prioritize and value sufficient and appropriately timed sleep. Future research to understand the molecular underpinnings of these findings (for example in circadian clocks [135]) is needed to develop countermeasures to reduce the impact of insufficient sleep, disrupted sleep, or circadian misalignment on cardiometabolic health. This is because insufficient sleep and night shift work may sometimes be unavoidable. Such investigations are currently being planned.
Peter Y. Liu, · Radha T. Reddy
Abstract
Sleep serves important biological functions and influences health and longevity through endocrine and metabolic-related systems. Sleep debt, circadian misalignment, and sleep disruption from obstructive sleep apnea are widespread in modern society and accumulate with life because recovery sleep is not completely restorative. Accumulated disordered sleep throughout life impacts the aging process and the development of age-related diseases. When epidemiological and interventional studies are considered collectively, sleep loss and lower sleep duration are associated with lower morning, afternoon, and 24-h testosterone; as well as higher afternoon, but not morning or 24-h cortisol. These reciprocal changes imbalance anabolic catabolic signaling because testosterone and cortisol are respectively the main anabolics and catabolic signals in man. Fixing testosterone-cortisol balance by means of a novel dual-hormone clamp mitigates the induction of insulin resistance by sleep restriction and provided the first proof of concept that the metabolic harm from sleep loss can be ameliorated by approaches that do not require sleeping more. Obstructive sleep apnea is associated with lower testosterone, even after controlling for age and obesity whereas the conclusion that continuous positive airway pressure therapy has no effect on testosterone is premature because available studies are underpowered and better-quality studies suggest otherwise. High-dose testosterone therapy induces OSA, but more physiological dosing may not, and this effect may be transient or may dissipate with longer-term therapy. Studies investigating the origin of the diurnal testosterone rhythm, the effect of circadian misalignment on testosterone-cortisol balance, and methods to mitigate metabolic harm, are required.
1 Introduction
Sleep restores neurobehavioral performance [1], improves immune function [2], and conserves whole-body energy expenditure through metabolic processes that also restore brain energy stores and are important for neuronal plasticity and connectivity [3]. These sleep-related benefits on metabolism, immunity, and cognition are essential for healthy aging. Conversely, the accumulation of sleep debt across the lifespan negatively impacts metabolic conservation, neurobehavioral performance, immunity, and autoimmunity – impacts that may be responsible for the development of diseases that accrue with age.
Sleep debt accumulates throughout life from repetitive episodes of insufficient sleep (not sleeping for an adequate amount of time), misaligned sleep (as occurs with jetlag or night shiftwork), and disrupted sleep (from obstructive sleep apnea, nocturia associated with aging, or sporadic environmental noise) [4]. This accumulation occurs because intermittent recovery or “catch-up” sleep does not seem to completely reverse the adverse effects of sleep debt on multiple physiological processes including psychomotor performance [1, 5], metabolism [6, 7], blood pressure regulation [8] and immune/adrenal response [9]. Furthermore, the accumulation of sleep debt is widespread in modern society: approximately one in three sleep insufficiently (<7 h per night) [10], up to 20% are shift workers with work schedules that misalign sleep [11], and 10% of men (3% of women) aged 30–49 years and 17% of men (9% of women) aged 50–70 years have at least moderate obstructive sleep apnea [12]. Insufficient, misaligned, and disrupted sleep is therefore likely to have major repercussions on aging and the development of age-related diseases because catch-up sleep is ineffectual and abnormal sleep is widespread in our modern society. An important consideration in determining accumulated sleep debt is that sleep need itself decrease with older age. Another consideration is that the COVID-19 pandemic increased the global prevalence of sleep disturbances, particularly in younger individuals infected with the disease, but whether this trend will persist is not yet known [13]
Sleep may influence health and longevity through endocrine and metabolic systems [14]. This is because endocrine networks evolved to regulate whole-body metabolism, including catabolism and anabolism, in a diurnally appropriate manner, and to simultaneously allow dynamic responses to external environmental insults and internal stress through the ultradian (also known as pulsatile) nature of hormone secretion [15, 16]. An important concept here is that the circadian pattern in hormones allows for anticipation of regular events that occur in a 24 h day (for example to regulate whole-body metabolism), whereas the pulsatile nature of hormone release results in large changes in hormone concentrations in a short period of time which allows for a more rapid and biologically efficient (as opposed to a sustained non-pulsatile release) response to unpredictable events. In this fashion, specific endocrine networks can coordinate growth, puberty, and reproduction in an age- and environment-appropriate manner.
*This review will primarily examine the effect of sleep disturbances on testosterone in the context of male gonadal aging. However, to understand the full impact of restricted sleep on testosterone, it is necessary to determine the net effects on whole body metabolism and metabolic illnesses in conjunction with cortisol: see Fig. 1. This is because the hypothalamic-pituitary testicular and hypothalamic-pituitary-adrenal axes respectively control testosterone, the major anabolic hormone in men (but not women) and cortisol, a key catabolic signal, are intertwined in their regulation and sleep restriction imbalances both their signaling [4]. Obstructive sleep apnea (OSA) and its interaction with testosterone are of particular clinical relevance because sleep architecture is disrupted and sleep duration is reduced. The review will therefore conclude with a discussion of OSA and testosterone in the context of aging.
2 Sleep, sleep architecture, and aging
3 The inter‑relationships between sleep duration, testosterone, and aging
3.1 Testosterone and aging
3.2 Sleep duration, testosterone, and aging
4 The inter‑relationships between sleep duration, cortisol, and aging
4.1 Cortisol and aging
4.2 Sleep duration, cortisol, and aging
5 Testosterone and cortisol imbalance from sleep restriction leads to metabolic harm
6 Effect of circadian misalignment on testosterone and cortisol
7 Obstructive sleep apnea
7.1 OSA, obesity, and testosterone in aging men
7.2 Testosterone effects on OSA
8 Summary and conclusions
Sleep is highly organized, serves important biological functions, and influences health and longevity through endocrine and metabolic-regulated systems. Accumulated sleep debt is widespread in modern society and when accumulated throughout life likely impacts the aging process, and the development of age-related diseases.
Sleep loss and lower sleep duration are associated with lower morning, afternoon, and 24-h testosterone, whereas they are associated with higher late afternoon and early evening, but not morning or 24-h cortisol. These reciprocal changes in testosterone and cortisol with sleep loss imbalances catabolic-anabolic signaling and is an important, but not exclusive, the mechanism by which sleep loss induces insulin resistance. By fixing testosterone-cortisol balance to prevent the induction of insulin resistance by sleep restriction, we provided the first proof of concept that the metabolic harm that occurs with sleep loss can potentially be mitigated by therapeutic approaches that do not require sleeping more. This approach is likely to be relevant also to older men since the changes in testosterone-cortisol balance that occur in young men have recently been shown to also occur in older men.
Epidemiological studies when considered in unison show that OSA is associated with lower testosterone levels, independently of confounders such as age and obesity. Circumstantial evidence would favor the possibility that more severe OSA, due to greater hypoxemia, lowers testosterone although the opposite is plausible. It would be premature to conclude that CPAP therapy has no effect on testosterone in men with OSA since the available studies are underpowered, and the higher quality studies suggest otherwise. In contrast, the larger number of studies available, particularly higher quality studies, has allowed the conclusion to be made that CPAP decreases cortisol. High-dose testosterone therapy induces OSA, but more physiological dosing may not; this effect may be transient or dissipate with longer-term therapy
Important limitations are that only one interventional study has examined the effect of restricting sleep on testosterone and cortisol in a cohort of older men; few studies have been designed to examine changes in relevant testosterone and cortisol pulse characteristics with age and/or in response to sleep manipulation; and longitudinal epidemiological studies examining the age-related changes in sleep architecture, the impact of changes in sleep on testosterone and cortisol, are rudimentary.
Nevertheless, the available data are highly suggestive that restricted sleep, circadian misalignment, and disrupted sleep from OSA are relevant to age and age-related diseases through alterations in testosterone and cortisol signaling. Therefore, society should prioritize and value sufficient and appropriately timed sleep. Future research to understand the molecular underpinnings of these findings (for example in circadian clocks [135]) is needed to develop countermeasures to reduce the impact of insufficient sleep, disrupted sleep, or circadian misalignment on cardiometabolic health. This is because insufficient sleep and night shift work may sometimes be unavoidable. Such investigations are currently being planned.

















