Nelson Vergel
Founder, ExcelMale.com
A study published at the end of 2013 and a previous one from the Veterans Administration Hospital System (see below post) show what happens when older men are given suboptimal testosterone replacement without proper monitoring and management of factors that can affect their health.
Testosterone replacement can increase red blood cells (and hematocrit which is the total red cell volume) and estradiol production, two important factors for men's health when present in normal levels (red blood cells carry oxygen and estradiol maintains healthy bones, cognitive function, and sex drive). However, due to genetics, age or other factors, some men can have excessive production of both. High hematocrit and estradiol are more common in older men on testosterone since testosterone-related aromatization and erythrocytosis are worse in that population. In excess, both variables have been linked to cardiovascular risks in older men. Not a single one of the studies reporting higher cardiovascular risks in men on testosterone have managed either important factors.
Additionally, this latest nonrandomized study states that "No data were available on indications for T prescription, race, laboratory findings, occupational, environmental, or lifestyle factors."
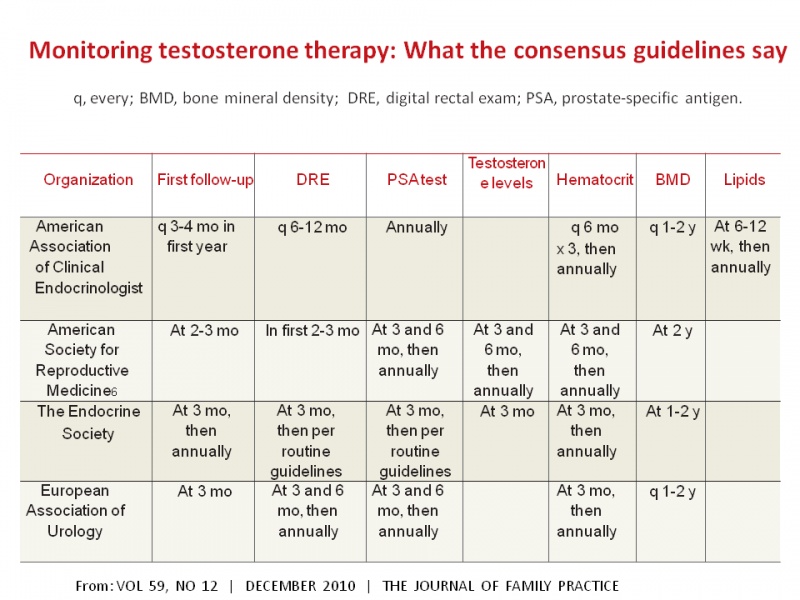
Many clinics are managing hematocrit by recommending blood donation or phlebotomies to men with a hematocrit over 53. They are also recommending treatment with low dose anastrozole for men with estradiol over 50 pg/mL. However, an alarming number of medical practices and research studies chose not to follow basic recommendations from the 4 current guideline groups that, in my opinion, may still need revision to add estradiol monitoring. The table below shows a summary of the monitoring required by the main 4 guidelines groups in the world.
It is also imperative that future studies at least follow the minimum requirements of the current guidelines. The last few studies that concluded that testosterone may increase cardiovascular risks did not monitor or report hematocrit blood levels. Most guidelines recommend monitoring hematocrit at months 3, 6 and then annually. Are these studies liable for not following minimum guidelines and exposing their volunteers to increased risks?. I do not why I do not see discussions on this alarming fact. Institutional review boards (IRB's) need to educate themselves about this problem so that no more studies are allowed that do not properly monitor men on testosterone replacement. Would a class action lawsuit be required to change this malpractice? Some are already popping up: Testosterone Treatment Lawsuit Information
It is time to revise the current guidelines for testosterone treatment in men to include monitoring and managing estradiol with the same frequency as hematocrit. It is also time to enforce guidelines compliance in all testosterone studies.
I encourage all men currently on testosterone replacement or thinking about starting it to familiarize themselves with the table below and demand that guidelines (along with estradiol monitoring) are followed by their physicians. And, please, do not sign a study consent form if you see that they do not specify the monitoring frequency and side effect management options the researchers are offering.
I also encourage Abbvie (makers of Androgel), Auxillum (makers of Testim), Endo Pharmaceuticals (makers of Fortesta), Lilly (makers of Axiron) to wake up to this fact before they lose millions due to negligent practices that do not follow minimum guidelines.
I am looking forward to the day when a research group will perform a good study that uses a protocol that not only gives testosterone to men but also one that retests them periodically to manage dose, high hematocrit, and estradiol blood levels. It should not be a difficult task and it is the only responsible thing to do to come up with conclusions that we can trust.
For options on how to prevent and reverse potential side effects of testosterone replacement: click here
Reference: Increased Risk of Non-Fatal Myocardial Infarction Following Testosterone Therapy Prescription in Men
Testosterone replacement can increase red blood cells (and hematocrit which is the total red cell volume) and estradiol production, two important factors for men's health when present in normal levels (red blood cells carry oxygen and estradiol maintains healthy bones, cognitive function, and sex drive). However, due to genetics, age or other factors, some men can have excessive production of both. High hematocrit and estradiol are more common in older men on testosterone since testosterone-related aromatization and erythrocytosis are worse in that population. In excess, both variables have been linked to cardiovascular risks in older men. Not a single one of the studies reporting higher cardiovascular risks in men on testosterone have managed either important factors.
Additionally, this latest nonrandomized study states that "No data were available on indications for T prescription, race, laboratory findings, occupational, environmental, or lifestyle factors."
Many clinics are managing hematocrit by recommending blood donation or phlebotomies to men with a hematocrit over 53. They are also recommending treatment with low dose anastrozole for men with estradiol over 50 pg/mL. However, an alarming number of medical practices and research studies chose not to follow basic recommendations from the 4 current guideline groups that, in my opinion, may still need revision to add estradiol monitoring. The table below shows a summary of the monitoring required by the main 4 guidelines groups in the world.
It is also imperative that future studies at least follow the minimum requirements of the current guidelines. The last few studies that concluded that testosterone may increase cardiovascular risks did not monitor or report hematocrit blood levels. Most guidelines recommend monitoring hematocrit at months 3, 6 and then annually. Are these studies liable for not following minimum guidelines and exposing their volunteers to increased risks?. I do not why I do not see discussions on this alarming fact. Institutional review boards (IRB's) need to educate themselves about this problem so that no more studies are allowed that do not properly monitor men on testosterone replacement. Would a class action lawsuit be required to change this malpractice? Some are already popping up: Testosterone Treatment Lawsuit Information
It is time to revise the current guidelines for testosterone treatment in men to include monitoring and managing estradiol with the same frequency as hematocrit. It is also time to enforce guidelines compliance in all testosterone studies.
I encourage all men currently on testosterone replacement or thinking about starting it to familiarize themselves with the table below and demand that guidelines (along with estradiol monitoring) are followed by their physicians. And, please, do not sign a study consent form if you see that they do not specify the monitoring frequency and side effect management options the researchers are offering.
I also encourage Abbvie (makers of Androgel), Auxillum (makers of Testim), Endo Pharmaceuticals (makers of Fortesta), Lilly (makers of Axiron) to wake up to this fact before they lose millions due to negligent practices that do not follow minimum guidelines.
I am looking forward to the day when a research group will perform a good study that uses a protocol that not only gives testosterone to men but also one that retests them periodically to manage dose, high hematocrit, and estradiol blood levels. It should not be a difficult task and it is the only responsible thing to do to come up with conclusions that we can trust.
For options on how to prevent and reverse potential side effects of testosterone replacement: click here
Reference: Increased Risk of Non-Fatal Myocardial Infarction Following Testosterone Therapy Prescription in Men
Last edited: