Nelson Vergel
Founder, ExcelMale.com

Trimel Pharmaceuticals Corporation announced today (May 28.2014) that the United States Food and Drug Administration (FDA) has approved Natesto (testosterone), formerly CompleoTRT, the first and only testosterone nasal gel for replacement therapy in adult males for conditions associated with a deficiency or absence of endogenous testosterone. Natesto is self-administered via a nasal applicator thereby minimizing the risk of secondary exposure to testosterone of women or children.
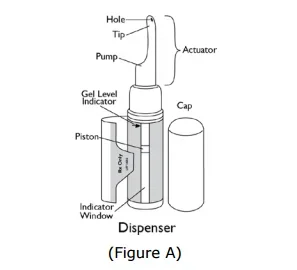
The product is self-administered into the nostrils via a metered-dose pump applicator. One pump actuation delivers 5.5 mg of testosterone, and the recommended dose is 11 mg (2 pump actuations, 1 in each nostril), 3 times daily (total 33 mg/day).
Bradley D. Anawalt, MD, chief of medicine at the University of Washington Medical Center, Seattle, and chair of the Hormone Health Network for the Endocrine Society, pointed to a theoretic concern, that brain testosterone levels will be much higher with intranasal testosterone gel than with other testosterone treatments. A study published in 2009 using mice showed brain levels of testosterone that were about twice as high in mice who received intranasal testosterone gel than in mice receiving intravenous testosterone. "What this finding means and whether it applies to men is unknown," he told Medscape Medical News.