Nelson Vergel
Founder, ExcelMale.com
Successful Percutaneous Dihydrotestosterone Treatment of Gynecomastia Occurring during Highly Active Antiretroviral Therapy: Four Cases and a Review of the Literature

Abstract
Fourteen cases of gynecomastia occurring during highly active antiretroviral therapy (HAART) have been reported in the literature. To date, no specific therapeutic approach has been proposed, and gynecomastia has usually persisted. We report 4 new cases of HAART-induced gynecomastia that were successfully treated with percutaneous dihydrotestosterone gel.
Fourteen cases of gynecomastia (GM) and 2 cases of breast hypertrophy in women [7, 8] occurring during highly active antiretroviral therapy (HAART) have been reported in the literature. For only 4 patients did GM resolve at some unspecified time or at 1, 5, or 7 months after changing the HAART regimen (table 1). For the 12 remaining patients, both men and women, breast hypertrophy persisted for many months and may have been responsible for one suicide [2].
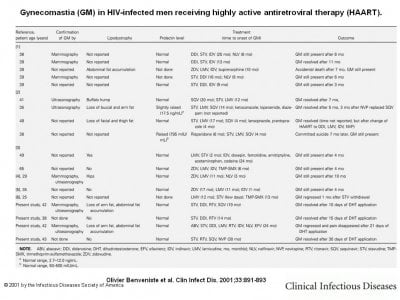
Gynecomastia (GM) in HIV-infected men receiving highly active antiretroviral therapy (HAART).
Dihydrotestosterone (DHT; Andractim [Besins-Iscovesco]) is a natural metabolite of testosterone that has antiestrogen activity and cannot be converted into estradiol at the tissue level. Systemic [9] or locally [10] administered DHT has been successfully prescribed for the treatment of patients with idiopathic and estrogen-induced GM. We report 4 new cases of GM occurring during HAART for which percutaneous DHT (androstanolone) gel application led to dramatic regression of breast enlargement.
Patient 1. A 42-year-old man had HIV infection diagnosed in 1986. In May 1996, therapy with stavudine (30 mg b.i.d.), lamivudine (150 mg b.i.d.), and ritonavir (600 mg b.i.d.) was begun. Because of digestive intolerance, the regimen was modified in March 1997 to stavudine (30 mg b.i.d.), didanosine (300 mg/day), ritonavir (100 mg b.i.d.), and saquinavir (400 mg b.i.d.). He developed lipodystrophy (defined as an abnormal fat distribution, either accumulation or loss, as specified in each case), particularly loss of fat from the arms and abdominal fat accumulation. In October 1998, the patient developed right side GM, which was confirmed by mammography and ultrasonography. Analysis of needle-puncture biopsy specimens showed normal ductal tissue. His CD4+ cell count was 411 cells/mm3 and his HIV load was <200 copies/mL. Prolactin, follicle-stimulating hormone, luteinizing hormone, estradiol, Δ4-androstenedione, and total testosterone levels were within normal ranges. In December 1998, he began local application of DHT gel to the breast (5 g of DHT per day). Within 10 days, GM disappeared, and he stopped DHT therapy 20 days later. In April 2000, he developed GM of the contralateral breast but declined treatment.
Patient 2. A 38-year-old man had HIV infection diagnosed in 1994. In August 1997, therapy with stavudine (40 mg b.i.d.), didanosine (400 mg/d), and ritonavir (600 mg b.i.d.) was begun. In October 1998, he developed bilateral GM. No lipodystrophy was observed. His CD4+ cell count was 887 cells/mm3 and his HIV load was <200 copies/mL. Prolactin, Δ4-androstenedione, and total testosterone levels were within normal ranges. In January 1999, he began local application of DHT gel to his breasts. Within 15 days, GM disappeared. He stopped DHT therapy 15 days later and has had no relapse to date.
Patient 3. A 42-year-old man had HIV infection diagnosed in 1987. In December 1994, he started therapy with zidovudine (250 mg b.i.d.), lamivudine (150 mg b.i.d.), and trimethoprimsulfamethoxazole (80 mg-400 mg/day). In March 1996, the regimen was changed to stavudine (40 mg b.i.d.), lamivudine, and ritonavir (600 mg b.i.d.). In July 1997, he developed disseminated tuberculosis, which was treated for 12 months with regimens based on isoniazid and rifabutin, plus pyrazinamide and ethambutol during the first 3 months. In October 1997, he began therapy with abacavir (300 mg b.i.d.), nelfinavir (750 mg t.i.d.), saquinavir (600 mg t.i.d.), and lamivudine (150 mg b.i.d.) until May 1998, when therapy with abacavir (300 mg b.i.d.), stavudine (40 mg b.i.d.), and didanosine (400 mg/day), lamivudine (150 mg b.i.d.) and ritonavir (100 mg b.i.d.), indinavir (400 mg b.i.d.), nelfinavir (750 mg t.i.d.) and efavirenz (600 mg/day) was begun. In May 2000, the patient presented with painful right-side GM, which was confirmed by mammography and ultrasonography. He developed minor lipodystrophy (loss of fat of the arms and accumulation of fat in the abdominal region). His CD4+ cell count was 631 cells/mm3 and his HIV load was <200 copies/mL. Prolactin, Δ4-androstenedione, and total testosterone levels were within normal ranges. He started local application of DHT gel to the breast. Within 21 days, pain disappeared and GM slightly regressed. He stopped the treatment 60 days later with the persistence of indolent attenuated GM.
Patient 4. A 43-year-old man had HIV infection diagnosed in 1988. In December 1994, he started receiving therapy with zidovudine (250 mg b.i.d.), didanosine (400 mg/d), and trimethoprim-sulfamethoxazole (80 mg–400 mg/day). In August 1996, the patient received zidovudine (250 mg b.i.d.), lamivudine (150 mg b.i.d.), and indinavir (800 mg t.i.d.) that was switched to nelfinavir (750 mg t.i.d.) in June 1997 because of crystalluria. In December 1997, he started therapy with stavudine (30 mg b.i.d.), ritonavir (100 mg b.i.d.), saquinavir (400 mg b.i.d.), and nevirapine (200 mg b.i.d.). In June 2000, the patient developed painful bilateral GM. No lipodystrophy was observed. His CD4+ cell count was 440 cells/mm3 and his HIV load was <200 copies/mL. Prolactin, Δ4-androstenedione, and total testosterone levels were within normal ranges. In July 2000, he started local application of DHT gel to the breasts. Within 10 days, pain disappeared and GM regressed. GM finally disappeared after 30 days. He stopped the treatment 30 days later and has had no relapse to date.
Discussion. Two mechanisms might explain the occurrence of breast enlargement among patients who are receiving HAART: (1) pseudo-GM, in which the enlargement is due to an increase of adipose tissue, and (2) real GM with glandular hypertrophy. Fat accumulation is a well-known phenomenon for patients receiving HAART that includes a protease inhibitor. Stavudine has also been reported to induce GM [6], but it is better known to cause fat atrophy [11]. Eight of the 18 men (table 1) had lipodystrophy with mostly fat accumulation, but true GM with glandular structures was confirmed by mammography, ultrasonography, or both for 8 of the 18 men (table 1). These findings prompted us to think that breast enlargement would be due to drugs having a mammotropic action, rather than to fat accumulation in the breasts. On the basis of our analysis of both the literature and our patients (table 1), no particular class of molecule included in HAART seems to be a particularly good candidate to explain this effect.
The mammotropic action of drugs can be divided into 2 main known types: those that mimic estrogen or progesterone at peripheral receptor sites (e.g., digitalis or spironolactone) and those that increase secretion of prolactin (e.g., phenothiazine derivatives or tricyclic antidepressants). The latter drugs cause breast hypertrophy in men and women in equal proportions, and in women, they also induce galactorrhea and amenorrhea. The 2 women [6, 7] exhibited neither these symptoms nor high prolactin levels, as did 13 of the 18 men (table 1). For the remaining 2 men with abnormally high prolactin levels, GM might have been caused by the neuroleptic therapy they received concomitantly (table 1).
More likely, the mechanism involved for HAART would be the mimicking of the effect of estrogen on breast tissue. In fact, drugs that use this mechanism fairly frequently cause GM in men, as for HAART. However, women rarely note or complain of breast enlargement, especially because none of these agents has ever been reported to induce lactation. Furthermore, the dramatic effect of DHT gel, which has an antiestrogen effect, reinforces this last hypothesis.
This hypothesis should be tested on a larger cohort of patients (e.g., in a controlled clinical trial); however, from a practical point of view, when such GM is observed in patients who are receiving HAART and when a careful search has been done to detect predisposing causes that should be specifically treated, rapid, beneficial symptomatic response can be obtained with local application of DHT gel, 5 g/day given once daily for 1–3 months. This simple treatment seems to be particularly well tolerated: no side effects have been reported in the literature [10] or were observed among the patients who we studied. Nevertheless, the anecdotal nature of the effect of DHT presented here prompts us to think that other systemic androgenic therapies (e.g., testosterone gel) might have the same effect.
References
Peyriere H, Mauboussin JM, Rouanet I, et al . Report of gynecomastia in five male patients during antiretroviral therapy for HIV infection. AIDS 1999;13:2167-9. CrossRefMedlineWeb of ScienceGoogle Scholar
Donovan B, Bodsworth NJ, Mulhall BP, et al . Gynaecomastia associated with saquinavir therapy. Int J STD AIDS 1999;10:49-50. Abstract/FREE Full Text
Caeiro JP, Visnegarwala F, Rodriguez-Barradas MC . Gynecomastia associated with indinavir therapy. Clin Infect Dis 1998;27:1539-40. FREE Full Text
Schurmann D, Bergmann F, Ehrenstein T, et al . Gynaecomastia in a male patient during protease inhibitor treatment for acute HIV disease. AIDS 1998;12:2232-3. MedlineWeb of ScienceGoogle Scholar
Toma E, Therrien R . Gynecomastia during indinavir antiretroviral therapy in HIV infection. AIDS 1998;12:681-2. MedlineWeb of ScienceGoogle Scholar
Melbourne KM, Brown SL, Silverblatt FJ . Gynecomastia with stavudine treatment in an HIV-positive patient. Ann Pharmacother 1998;32:1108. FREE Full Text
Herry I, Bernard L, Truchis P, et al . Hypertrophy of the breasts in a patient treated with indinavir. Clin Infect Dis 1997;25:937-8. FREE Full Text
Lui A, Karter D, Turett G . Another case of breast hypertrophy in a patient treated with indinavir. Clin Infect Dis 1998;26:1482. FREE Full Text
Eberle AJ, Sparrow JT, Keenan BS . Treatment of persistent pubertal gynecomastia with dihydrotestosterone heptanoate. J Pediatr 1986;109:144-9. CrossRefMedlineWeb of ScienceGoogle Scholar
Kuhn JM, Roca R, Laudat MH, et al . Studies on the treatment of idiopathic gynaecomastia with percutaneous dihydrotestosterone. Clin Endocrinol (Oxford) 1983;19:513-20. MedlineGoogle Scholar
Saint-Marc T, Partisani M, Poizot-Martin I, et al . Fat distribution evaluated by computed tomography and metabolic abnormalities in patients undergoing antiretroviral therapy: preliminary results of the LIPOCO study. AIDS 2000;14:37-49. CrossRefMedlineWeb of ScienceGoogle Scholar

Abstract
Fourteen cases of gynecomastia occurring during highly active antiretroviral therapy (HAART) have been reported in the literature. To date, no specific therapeutic approach has been proposed, and gynecomastia has usually persisted. We report 4 new cases of HAART-induced gynecomastia that were successfully treated with percutaneous dihydrotestosterone gel.
Fourteen cases of gynecomastia (GM) and 2 cases of breast hypertrophy in women [7, 8] occurring during highly active antiretroviral therapy (HAART) have been reported in the literature. For only 4 patients did GM resolve at some unspecified time or at 1, 5, or 7 months after changing the HAART regimen (table 1). For the 12 remaining patients, both men and women, breast hypertrophy persisted for many months and may have been responsible for one suicide [2].

Gynecomastia (GM) in HIV-infected men receiving highly active antiretroviral therapy (HAART).
Dihydrotestosterone (DHT; Andractim [Besins-Iscovesco]) is a natural metabolite of testosterone that has antiestrogen activity and cannot be converted into estradiol at the tissue level. Systemic [9] or locally [10] administered DHT has been successfully prescribed for the treatment of patients with idiopathic and estrogen-induced GM. We report 4 new cases of GM occurring during HAART for which percutaneous DHT (androstanolone) gel application led to dramatic regression of breast enlargement.
Patient 1. A 42-year-old man had HIV infection diagnosed in 1986. In May 1996, therapy with stavudine (30 mg b.i.d.), lamivudine (150 mg b.i.d.), and ritonavir (600 mg b.i.d.) was begun. Because of digestive intolerance, the regimen was modified in March 1997 to stavudine (30 mg b.i.d.), didanosine (300 mg/day), ritonavir (100 mg b.i.d.), and saquinavir (400 mg b.i.d.). He developed lipodystrophy (defined as an abnormal fat distribution, either accumulation or loss, as specified in each case), particularly loss of fat from the arms and abdominal fat accumulation. In October 1998, the patient developed right side GM, which was confirmed by mammography and ultrasonography. Analysis of needle-puncture biopsy specimens showed normal ductal tissue. His CD4+ cell count was 411 cells/mm3 and his HIV load was <200 copies/mL. Prolactin, follicle-stimulating hormone, luteinizing hormone, estradiol, Δ4-androstenedione, and total testosterone levels were within normal ranges. In December 1998, he began local application of DHT gel to the breast (5 g of DHT per day). Within 10 days, GM disappeared, and he stopped DHT therapy 20 days later. In April 2000, he developed GM of the contralateral breast but declined treatment.
Patient 2. A 38-year-old man had HIV infection diagnosed in 1994. In August 1997, therapy with stavudine (40 mg b.i.d.), didanosine (400 mg/d), and ritonavir (600 mg b.i.d.) was begun. In October 1998, he developed bilateral GM. No lipodystrophy was observed. His CD4+ cell count was 887 cells/mm3 and his HIV load was <200 copies/mL. Prolactin, Δ4-androstenedione, and total testosterone levels were within normal ranges. In January 1999, he began local application of DHT gel to his breasts. Within 15 days, GM disappeared. He stopped DHT therapy 15 days later and has had no relapse to date.
Patient 3. A 42-year-old man had HIV infection diagnosed in 1987. In December 1994, he started therapy with zidovudine (250 mg b.i.d.), lamivudine (150 mg b.i.d.), and trimethoprimsulfamethoxazole (80 mg-400 mg/day). In March 1996, the regimen was changed to stavudine (40 mg b.i.d.), lamivudine, and ritonavir (600 mg b.i.d.). In July 1997, he developed disseminated tuberculosis, which was treated for 12 months with regimens based on isoniazid and rifabutin, plus pyrazinamide and ethambutol during the first 3 months. In October 1997, he began therapy with abacavir (300 mg b.i.d.), nelfinavir (750 mg t.i.d.), saquinavir (600 mg t.i.d.), and lamivudine (150 mg b.i.d.) until May 1998, when therapy with abacavir (300 mg b.i.d.), stavudine (40 mg b.i.d.), and didanosine (400 mg/day), lamivudine (150 mg b.i.d.) and ritonavir (100 mg b.i.d.), indinavir (400 mg b.i.d.), nelfinavir (750 mg t.i.d.) and efavirenz (600 mg/day) was begun. In May 2000, the patient presented with painful right-side GM, which was confirmed by mammography and ultrasonography. He developed minor lipodystrophy (loss of fat of the arms and accumulation of fat in the abdominal region). His CD4+ cell count was 631 cells/mm3 and his HIV load was <200 copies/mL. Prolactin, Δ4-androstenedione, and total testosterone levels were within normal ranges. He started local application of DHT gel to the breast. Within 21 days, pain disappeared and GM slightly regressed. He stopped the treatment 60 days later with the persistence of indolent attenuated GM.
Patient 4. A 43-year-old man had HIV infection diagnosed in 1988. In December 1994, he started receiving therapy with zidovudine (250 mg b.i.d.), didanosine (400 mg/d), and trimethoprim-sulfamethoxazole (80 mg–400 mg/day). In August 1996, the patient received zidovudine (250 mg b.i.d.), lamivudine (150 mg b.i.d.), and indinavir (800 mg t.i.d.) that was switched to nelfinavir (750 mg t.i.d.) in June 1997 because of crystalluria. In December 1997, he started therapy with stavudine (30 mg b.i.d.), ritonavir (100 mg b.i.d.), saquinavir (400 mg b.i.d.), and nevirapine (200 mg b.i.d.). In June 2000, the patient developed painful bilateral GM. No lipodystrophy was observed. His CD4+ cell count was 440 cells/mm3 and his HIV load was <200 copies/mL. Prolactin, Δ4-androstenedione, and total testosterone levels were within normal ranges. In July 2000, he started local application of DHT gel to the breasts. Within 10 days, pain disappeared and GM regressed. GM finally disappeared after 30 days. He stopped the treatment 30 days later and has had no relapse to date.
Discussion. Two mechanisms might explain the occurrence of breast enlargement among patients who are receiving HAART: (1) pseudo-GM, in which the enlargement is due to an increase of adipose tissue, and (2) real GM with glandular hypertrophy. Fat accumulation is a well-known phenomenon for patients receiving HAART that includes a protease inhibitor. Stavudine has also been reported to induce GM [6], but it is better known to cause fat atrophy [11]. Eight of the 18 men (table 1) had lipodystrophy with mostly fat accumulation, but true GM with glandular structures was confirmed by mammography, ultrasonography, or both for 8 of the 18 men (table 1). These findings prompted us to think that breast enlargement would be due to drugs having a mammotropic action, rather than to fat accumulation in the breasts. On the basis of our analysis of both the literature and our patients (table 1), no particular class of molecule included in HAART seems to be a particularly good candidate to explain this effect.
The mammotropic action of drugs can be divided into 2 main known types: those that mimic estrogen or progesterone at peripheral receptor sites (e.g., digitalis or spironolactone) and those that increase secretion of prolactin (e.g., phenothiazine derivatives or tricyclic antidepressants). The latter drugs cause breast hypertrophy in men and women in equal proportions, and in women, they also induce galactorrhea and amenorrhea. The 2 women [6, 7] exhibited neither these symptoms nor high prolactin levels, as did 13 of the 18 men (table 1). For the remaining 2 men with abnormally high prolactin levels, GM might have been caused by the neuroleptic therapy they received concomitantly (table 1).
More likely, the mechanism involved for HAART would be the mimicking of the effect of estrogen on breast tissue. In fact, drugs that use this mechanism fairly frequently cause GM in men, as for HAART. However, women rarely note or complain of breast enlargement, especially because none of these agents has ever been reported to induce lactation. Furthermore, the dramatic effect of DHT gel, which has an antiestrogen effect, reinforces this last hypothesis.
This hypothesis should be tested on a larger cohort of patients (e.g., in a controlled clinical trial); however, from a practical point of view, when such GM is observed in patients who are receiving HAART and when a careful search has been done to detect predisposing causes that should be specifically treated, rapid, beneficial symptomatic response can be obtained with local application of DHT gel, 5 g/day given once daily for 1–3 months. This simple treatment seems to be particularly well tolerated: no side effects have been reported in the literature [10] or were observed among the patients who we studied. Nevertheless, the anecdotal nature of the effect of DHT presented here prompts us to think that other systemic androgenic therapies (e.g., testosterone gel) might have the same effect.
References
Peyriere H, Mauboussin JM, Rouanet I, et al . Report of gynecomastia in five male patients during antiretroviral therapy for HIV infection. AIDS 1999;13:2167-9. CrossRefMedlineWeb of ScienceGoogle Scholar
Donovan B, Bodsworth NJ, Mulhall BP, et al . Gynaecomastia associated with saquinavir therapy. Int J STD AIDS 1999;10:49-50. Abstract/FREE Full Text
Caeiro JP, Visnegarwala F, Rodriguez-Barradas MC . Gynecomastia associated with indinavir therapy. Clin Infect Dis 1998;27:1539-40. FREE Full Text
Schurmann D, Bergmann F, Ehrenstein T, et al . Gynaecomastia in a male patient during protease inhibitor treatment for acute HIV disease. AIDS 1998;12:2232-3. MedlineWeb of ScienceGoogle Scholar
Toma E, Therrien R . Gynecomastia during indinavir antiretroviral therapy in HIV infection. AIDS 1998;12:681-2. MedlineWeb of ScienceGoogle Scholar
Melbourne KM, Brown SL, Silverblatt FJ . Gynecomastia with stavudine treatment in an HIV-positive patient. Ann Pharmacother 1998;32:1108. FREE Full Text
Herry I, Bernard L, Truchis P, et al . Hypertrophy of the breasts in a patient treated with indinavir. Clin Infect Dis 1997;25:937-8. FREE Full Text
Lui A, Karter D, Turett G . Another case of breast hypertrophy in a patient treated with indinavir. Clin Infect Dis 1998;26:1482. FREE Full Text
Eberle AJ, Sparrow JT, Keenan BS . Treatment of persistent pubertal gynecomastia with dihydrotestosterone heptanoate. J Pediatr 1986;109:144-9. CrossRefMedlineWeb of ScienceGoogle Scholar
Kuhn JM, Roca R, Laudat MH, et al . Studies on the treatment of idiopathic gynaecomastia with percutaneous dihydrotestosterone. Clin Endocrinol (Oxford) 1983;19:513-20. MedlineGoogle Scholar
Saint-Marc T, Partisani M, Poizot-Martin I, et al . Fat distribution evaluated by computed tomography and metabolic abnormalities in patients undergoing antiretroviral therapy: preliminary results of the LIPOCO study. AIDS 2000;14:37-49. CrossRefMedlineWeb of ScienceGoogle Scholar
Last edited:
















