* Use of non-proprietary testosterone cypionate as TRT results in an elevation in serum testosterone with favorable side effect profiles and overall patient satisfaction.
Who knew, LOL!
* Lastly, a cost analysis using the average available GoodRx prices in the Chicagoland area showed that a 1-month supply of non-proprietary testosterone cypionate was $235.45 less than Xyosted (the only FDA-approved SubQ TRT for management of male hypogonadism).
Non-proprietary Subcutaneous Testosterone Replacement Therapy for Hypogonadal Men
Mossack, S1; Spellman, A2; Desouky, E1; Nabulsi, O1; Loos, D1; Levine, L1
1 - Rush University Medical Center
2 - Mount Sinai Hospital
Introduction:
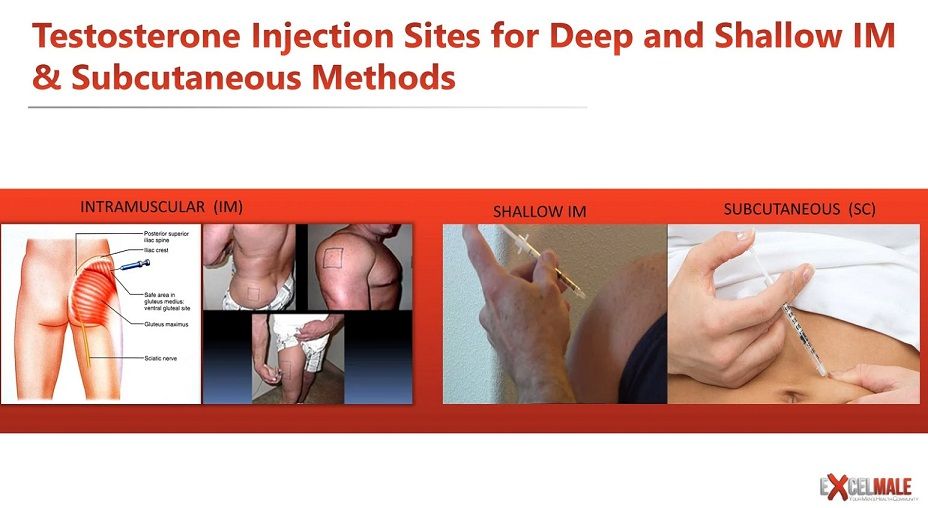
Advancement in the treatment for male hypogonadism involves investigation into safer, less expensive, and more effective alternatives than currently available treatment modalities, with subcutaneous testosterone replacement therapy (SubQ TRT) offering an attractive solution to several patient concerns.
Objective:
To assess the use of non-proprietary SubQ TRT as treatment for hypogonadal men and its effect on HCT, PSA, and patient satisfaction.
Methods:
This is a retrospective chart review of 93 men, mean age 60.6 years, receiving SubQ TRT with a mean follow up length of 17.9 months (range 0-52 months). Patient data was collected prior to initiation of therapy and at subsequent visits. Serum testosterone, HCT, and PSA were determined through regular blood draws, and patient demographics, AUA Symptom Index (AUA SI), Sexual Health Inventory for Men (SHIM) Scores, and patient satisfaction were collected at patient visits or via patient communication through email and phone. Univariate analysis and paired t-tests were used to analyze study variables.
Results:
93 patients received SubQ TRT. Mean changes in total testosterone level (increased 125.77 ng/dL, p-value<0.001), hematocrit (increased 1.2%), PSA (increased 0.3 ng/dl), SHIM score (increased 0.9 points) and AUA symptom score (decreased 0.3 points) were calculated at the patients’ initial and follow up visits while on SubQ TRT. Sixty-eight (73%) patients had been on various combinations of TRT before switching to SubQ: IM, CC, Testopel, T-Gel, HCG, and Anastrozole. Patient satisfaction surveys were completed by 57 patients (61%), with 26 (78.9%) patients reporting satisfaction with SubQ TRT versus 28.1% with past forms of TRT. Patients reported SubQ TRT to be both easier (71.4%) and generally preferred (71.1%) over their previous form of TRT. Several patients stopped SubQ TRT including one patient with stroke. Lastly, a cost analysis using the average available GoodRx prices in the Chicagoland area showed that a 1-month supply of non-proprietary testosterone cypionate was $235.45 less than Xyosted (the only FDA-approved SubQ TRT for management of male hypogonadism).
Conclusions:
Use of non-proprietary testosterone cypionate as TRT results in an elevation in serum testosterone with favorable side effect profiles and overall patient satisfaction.
Disclosure:
Any of the authors act as a consultant, employee or shareholder of an industry for: Consultant to Absorption Pharmaceuticals, Boston Scientific, Hybrid Medical, International Medical Devices and Irrimax Corp





Who knew, LOL!
* Lastly, a cost analysis using the average available GoodRx prices in the Chicagoland area showed that a 1-month supply of non-proprietary testosterone cypionate was $235.45 less than Xyosted (the only FDA-approved SubQ TRT for management of male hypogonadism).
Non-proprietary Subcutaneous Testosterone Replacement Therapy for Hypogonadal Men
Mossack, S1; Spellman, A2; Desouky, E1; Nabulsi, O1; Loos, D1; Levine, L1
1 - Rush University Medical Center
2 - Mount Sinai Hospital
Introduction:
Advancement in the treatment for male hypogonadism involves investigation into safer, less expensive, and more effective alternatives than currently available treatment modalities, with subcutaneous testosterone replacement therapy (SubQ TRT) offering an attractive solution to several patient concerns.
Objective:
To assess the use of non-proprietary SubQ TRT as treatment for hypogonadal men and its effect on HCT, PSA, and patient satisfaction.
Methods:
This is a retrospective chart review of 93 men, mean age 60.6 years, receiving SubQ TRT with a mean follow up length of 17.9 months (range 0-52 months). Patient data was collected prior to initiation of therapy and at subsequent visits. Serum testosterone, HCT, and PSA were determined through regular blood draws, and patient demographics, AUA Symptom Index (AUA SI), Sexual Health Inventory for Men (SHIM) Scores, and patient satisfaction were collected at patient visits or via patient communication through email and phone. Univariate analysis and paired t-tests were used to analyze study variables.
Results:
93 patients received SubQ TRT. Mean changes in total testosterone level (increased 125.77 ng/dL, p-value<0.001), hematocrit (increased 1.2%), PSA (increased 0.3 ng/dl), SHIM score (increased 0.9 points) and AUA symptom score (decreased 0.3 points) were calculated at the patients’ initial and follow up visits while on SubQ TRT. Sixty-eight (73%) patients had been on various combinations of TRT before switching to SubQ: IM, CC, Testopel, T-Gel, HCG, and Anastrozole. Patient satisfaction surveys were completed by 57 patients (61%), with 26 (78.9%) patients reporting satisfaction with SubQ TRT versus 28.1% with past forms of TRT. Patients reported SubQ TRT to be both easier (71.4%) and generally preferred (71.1%) over their previous form of TRT. Several patients stopped SubQ TRT including one patient with stroke. Lastly, a cost analysis using the average available GoodRx prices in the Chicagoland area showed that a 1-month supply of non-proprietary testosterone cypionate was $235.45 less than Xyosted (the only FDA-approved SubQ TRT for management of male hypogonadism).
Conclusions:
Use of non-proprietary testosterone cypionate as TRT results in an elevation in serum testosterone with favorable side effect profiles and overall patient satisfaction.
Disclosure:
Any of the authors act as a consultant, employee or shareholder of an industry for: Consultant to Absorption Pharmaceuticals, Boston Scientific, Hybrid Medical, International Medical Devices and Irrimax Corp