You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
TLANDO & TLANDO XR - novel oral prodrugs of testosterone
- Thread starter madman
- Start date
Nelson Vergel
Founder, ExcelMale.com
Nelson Vergel
Founder, ExcelMale.com
on Nov 2019:

 www.excelmale.com
www.excelmale.com
March 2020:

 www.excelmale.com
www.excelmale.com

Drug Application for Tlando Rejected Again by FDA
Tlando is an oral form of testosterone undecanoate and failed to achieve high enough blood testosterone levels at the recommended dosage. The article says: The FDA has turned down Lipocine Inc.'s Tlando, an oral testosterone product candidate for testosterone replacement therapy in adult males...
March 2020:

Pharma Company That Makes Jatenzo Sued
Clarus, the pharmaceutical company that makes Jatenzo, a recently introduced oral formulation of testosterone, has been sued by Lipocine, the company that makes Tlando, a rival oral formulation candidate, for patent violations. Tlando has been denied approval for sale three times by the FDA, but...
madman
Super Moderator
PIONEERING FOR NEXT-GENERATION ORAL TRT
TLANDO XR is a next-generation, novel ester prodrug of testosterone that uses the patent-protected Lip'ral technology to enhance solubility and improve systemic absorption. Lipocine completed a Phase 2b dose-finding study in hypogonadal men in 2016. The primary objectives of the Phase 2b clinical study were to determine the starting Phase 3 dose of TLANDO XR along with the safety and tolerability of TLANDO XR and its metabolites following oral administration of single and multiple doses in hypogonadal men. The Phase 2b clinical trial was a randomized, open-label, two-period, multi-dose PK study. Results suggested that the primary objectives were met, including identifying the dose expected to be tested in the planned Phase 3 study. A good dose-response relationship was observed over the tested dose range. Additionally, the target Phase 3 dose met primary and secondary endpoints. TLANDO XR was well tolerated with no drug-related severe or serious adverse events reported in the Phase 2b study.
Joe Sixpack
Active Member
This probably won't be a cheap drug.
madman
Super Moderator

Oral Prodrug of Testosterone Fast-Tracked for Nonalcoholic Steatohepatitis
https://www.empr.com/home/news/drugs-in-the-pipeline/oral-prodrug-of-testosterone-fast-tracked-for-nonalcoholic-steatohepatitis/ The Food and Drug Administration (FDA) has granted Fast Track designation to LPCN 1144 for the treatment of noncirrhotic nonalcoholic steatohepatitis (NASH). LPCN...
Nelson Vergel
Founder, ExcelMale.com
Their pipeline chart says the XR version has finished phase 2.
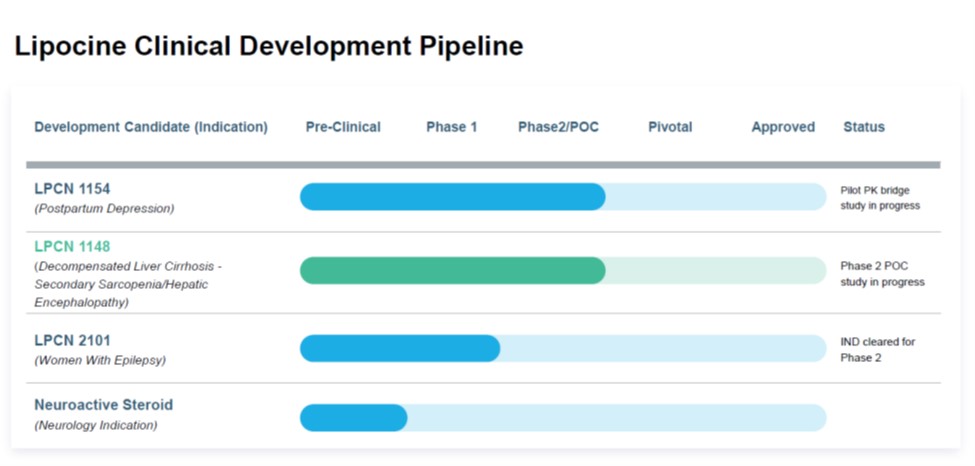
 www.lipocine.com
www.lipocine.com

Pipeline - Lipocine
Proprietary Solubilization Technology ENTAILS REPOSITIONING OF ESTABLISHED DRUGS WITH SIGNIFICANTLY IMPROVED PATIENT COMPLIANCE. Lipocine pipeline products are based on its … Pipeline Read More »
madman
Super Moderator
@madman, any idea if Tlando XR is currently in phase 3 studies?
Going to be a while!
TLANDO
Tlando was launched by partner Halozyme during the second quarter, which acquired original license holder Antares Pharma in May 2022. Halozyme announced the launch of the product in a June 7th press release and plans to commercialize Tlando along with Antares’ injectable testosterone replacement therapy Xyosted. Halozyme’s orientation as a commercialization company rather than a development company led to the passing of the Tlando XR (LPCN 1111) option. Tlando XR is now available for partnering. Lipocine is working with a contract manufacturer for the scale-up and manufacturing of LPCN 1111 and has completed a technology transfer to this end. Lipocine will not develop the product any further but is seeking a partner to do so and preparing for an efficient handoff with the product ready for clinical trials.
Systemlord
Member
When more oral T replacement comes out driving up competition and therefore making it more affordable, pellet therapy will be all but pointless.
The injections will still be king of being more convenient provided you're not on a daily protocol.
The injections will still be king of being more convenient provided you're not on a daily protocol.
Last edited:
Tlando appears to be an advantage over Jatenzo in that it does not require dose titration (not sure how this is the case) and fat content has less impact on absorption.
However, when looking at the pharmacokinetic curves, it appears that Tlando reaches peak levels around 6 hours after dosing, compared with two hours for Jatenzo. If you take Tlando at 6AM and 6PM, this would mean you would get a surge around midnight.
It seems wasteful to have those levels while you are sleeping, not to mention potentially disruptive to sleep.
However, when looking at the pharmacokinetic curves, it appears that Tlando reaches peak levels around 6 hours after dosing, compared with two hours for Jatenzo. If you take Tlando at 6AM and 6PM, this would mean you would get a surge around midnight.
It seems wasteful to have those levels while you are sleeping, not to mention potentially disruptive to sleep.
Online statistics
- Members online
- 7
- Guests online
- 8
- Total visitors
- 15
Totals may include hidden visitors.
© Copyright ExcelMale
















