madman
Super Moderator
ABSTRACT
Introduction: Patients with testosterone deficiency (TD) can be treated with exogenous testosterone (T) to achieve and maintain physiologic T levels and prevent negative clinical symptoms; with many testosterone replacement therapies currently available, this registration safety study was conducted to further characterize the clinical profile of chronically administered, concentration-guided subcutaneous testosterone enanthate (TE) dosing.
Aim: The purpose of this study was to confirm the safety and characterize the pharmacokinetic (PK) profile of the subcutaneous TE auto-injector (SCTE-AI) in adult men with TD.
Methods: In this phase III, 26-week study, 133 men 18-75 years of age with symptomatic TD self-administered SCTE-AI 75 mg once weekly for 6 weeks from July 2015 to June 2016. Dosing was adjusted when indicated to 50 mg or 100 mg to maintain T trough levels between 350 and 650 ng/dL (12.1-22.5 nmol/L). PK data were collected from a subgroup of patients receiving 75 mg SCTE-AI through week 12. Safety, including ambulatory blood pressure monitoring (ABPM), lipid levels, and adverse drug reactions, and PK were assessed
Results: In total, 34 patients (25.6%) experienced adverse drug reactions; the most frequently reported were increased hematocrit (52%) in 10 patients (7.5%), injection-site hemorrhage in 6 patients (4.5%), injection site bruising in 4 patients (3.0%), and increased prostate-specific antigen in 4 patients (3.0%). By week 26, mean systolic and diastolic blood pressure (BP) measured in the clinic increased by 3.4 mmHg (125.6-129.0 mmHg) and 1.8 mmHg (78.2-80.0 mmHg), respectively, from baseline. At week 12, ABPM showed 24-hour mean systolic and diastolic BP increases of 3.7 mmHg and 1.3 mmHg, respectively. All measured lipid fractions were below baseline levels at week 26. T, TE, dihydrotestosterone, and estradiol increased from weeks 1-12. T trough levels ranged from 300-650 ng/dL (10.4-22.5 nmol/L) in 82.4% and 83.2% of patients at weeks 12 and 26, respectively. Of the 965 assessed injections, mild pain was reported by 1 patient.
Clinical Implications: Dosing with SCTE is well-tolerated overall, yet associated with a numerically small mean systolic BP increase
Strengths & Implications: This study used a standardized ABPM protocol, confirming a numerically small systolic BP increase may be associated with reintroducing therapeutic T exposure in hypogonadal men. It is unknown at this time whether this applies with all routes of T supplementation.
Conclusion: SCTE-AI has a favorable safety profile and is well-tolerated, with a stable PK profile.
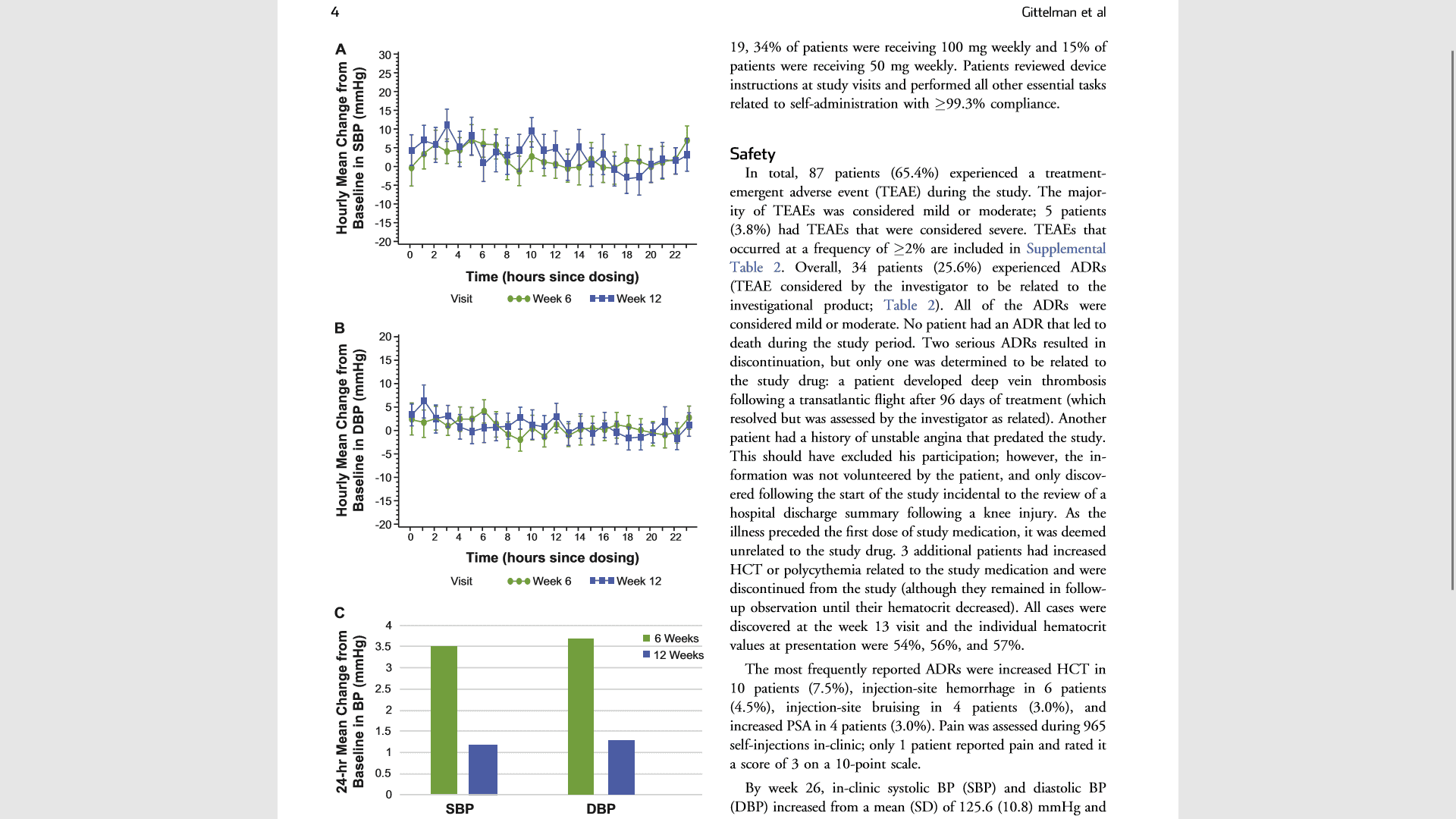
Figure 1. Hourly mean changes from baseline in panel A SBP and panel B DBP in the safety population and panel C is the 24-hour mean change in SBP and DBP from baseline at 6 and 12 weeks. Treatment with SCTE-AI resulted in nonsignificant changes in both SBP and DBP. DBP ¼ diastolic blood pressure; SBP ¼ systolic blood pressure; SCTE-AI ¼ subcutaneous testosterone enanthate auto-injector.
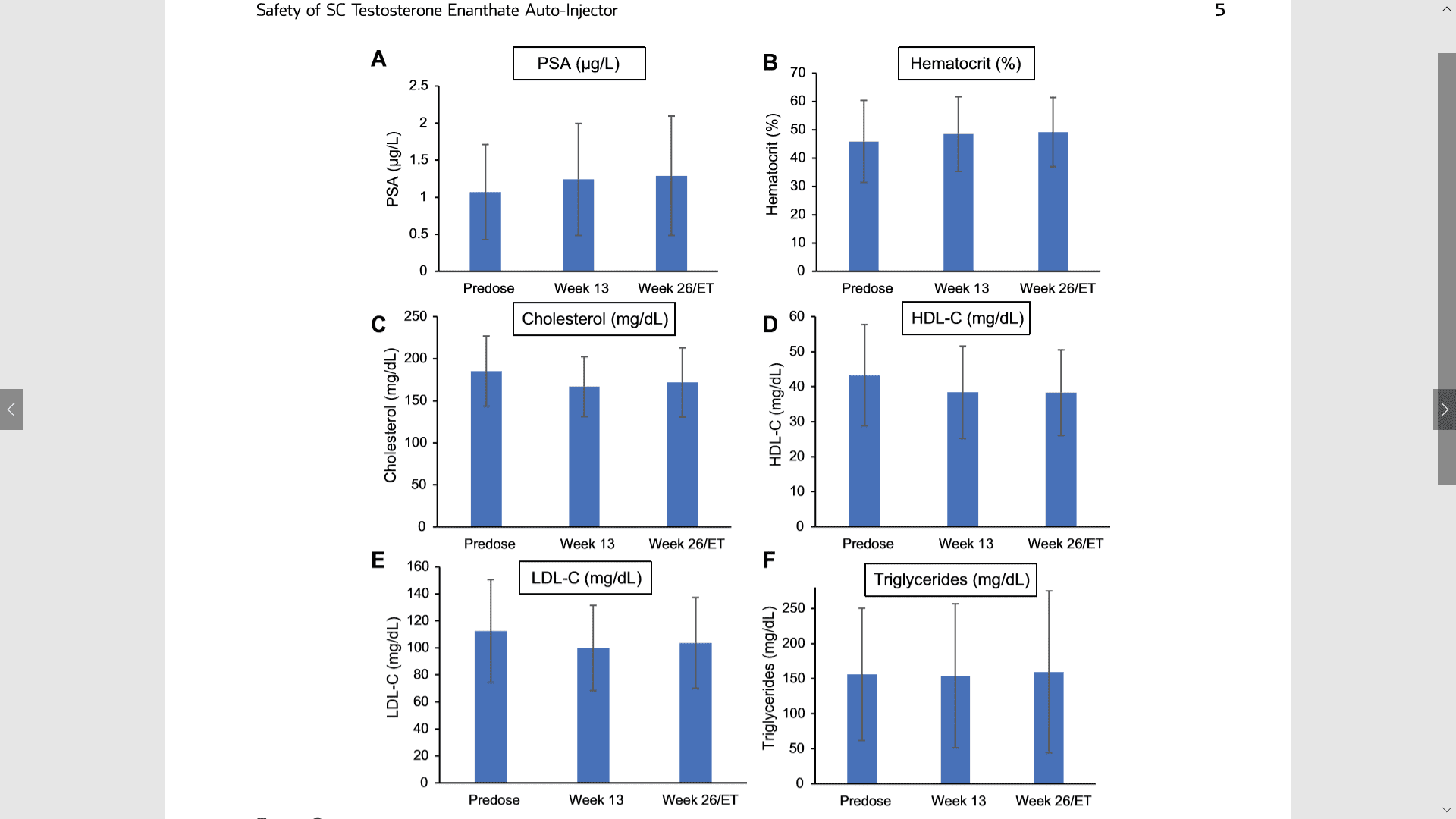
Figure 2. Clinical laboratory values for the safety population: panel A shows the PSA (mg/L); panel B shows the hematocrit (%); panel C shows the total cholesterol (mg/dL); panel D shows the HDL-C (mg/dL); panel E shows the LDL-C (mg/dL); and panel F shows the triglycerides (mg/dL). Values are expressed as mean (SD). HDL-C ¼ high-density lipoprotein cholesterol; LDL-C ¼ low-density lipoprotein cholesterol; PSA ¼ prostate-specific antigen.
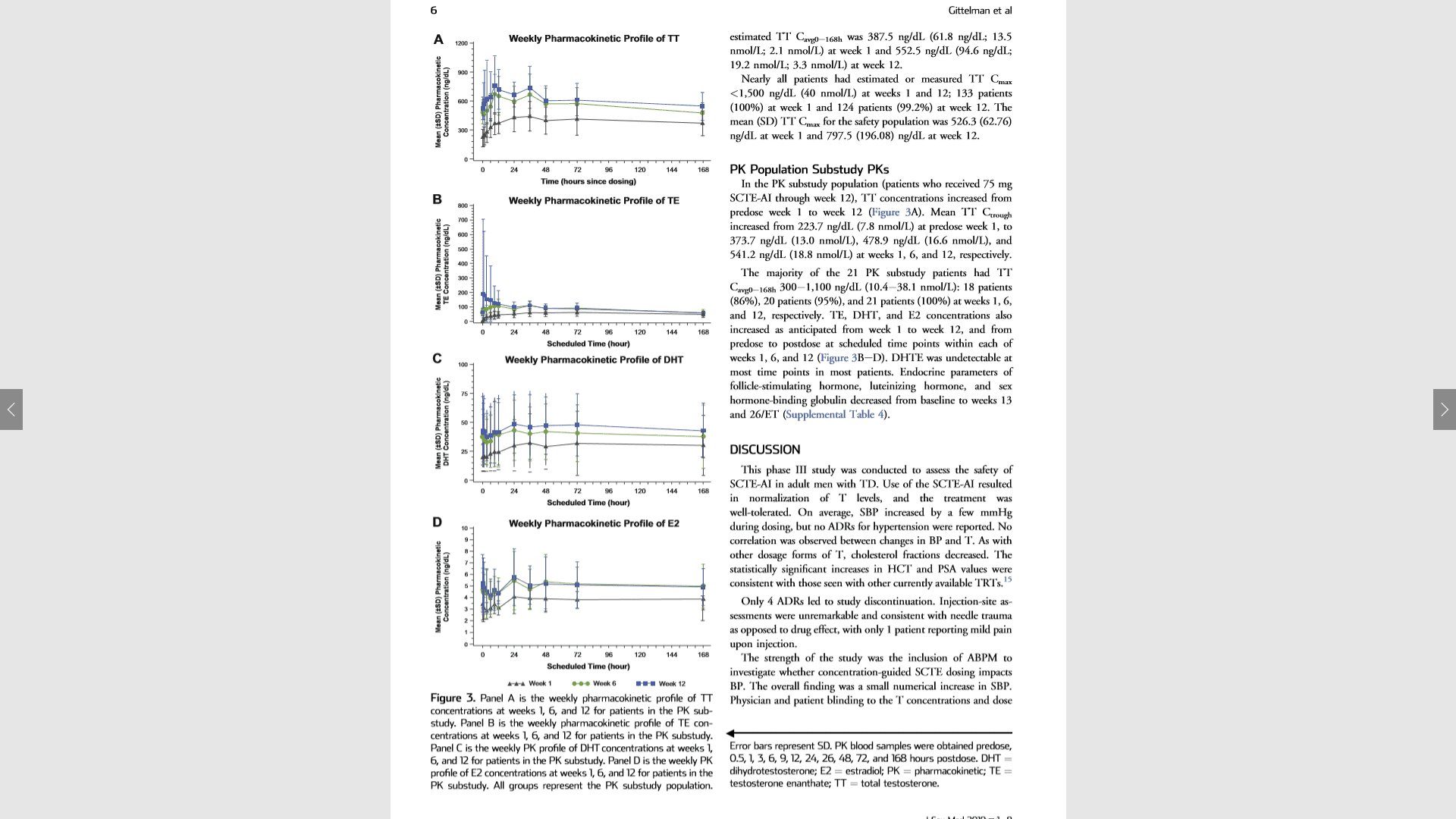
Figure 3. Panel A is the weekly pharmacokinetic profile of TT concentrations at weeks 1, 6, and 12 for patients in the PK substudy. Panel B is the weekly pharmacokinetic profile of TE concentrations at weeks 1, 6, and 12 for patients in the PK substudy. Panel C is the weekly PK profile of DHT concentrations at weeks 1, 6, and 12 for patients in the PK substudy. Panel D is the weekly PK profile of E2 concentrations at weeks 1, 6, and 12 for patients in the PK substudy. All groups represent the PK substudy population.
CONCLUSIONS
Overall, when considering clinical laboratory assessments, vital signs, clinical findings, and ADRs, this study demonstrates that once-weekly use of SCTE-AI in patients with TD is well tolerated and has a safety profile similar to other T products. Patients in the study were highly compliant and successfully self administered weekly injections with few remarkable injection-site reactions and reports of pain.
Introduction: Patients with testosterone deficiency (TD) can be treated with exogenous testosterone (T) to achieve and maintain physiologic T levels and prevent negative clinical symptoms; with many testosterone replacement therapies currently available, this registration safety study was conducted to further characterize the clinical profile of chronically administered, concentration-guided subcutaneous testosterone enanthate (TE) dosing.
Aim: The purpose of this study was to confirm the safety and characterize the pharmacokinetic (PK) profile of the subcutaneous TE auto-injector (SCTE-AI) in adult men with TD.
Methods: In this phase III, 26-week study, 133 men 18-75 years of age with symptomatic TD self-administered SCTE-AI 75 mg once weekly for 6 weeks from July 2015 to June 2016. Dosing was adjusted when indicated to 50 mg or 100 mg to maintain T trough levels between 350 and 650 ng/dL (12.1-22.5 nmol/L). PK data were collected from a subgroup of patients receiving 75 mg SCTE-AI through week 12. Safety, including ambulatory blood pressure monitoring (ABPM), lipid levels, and adverse drug reactions, and PK were assessed
Results: In total, 34 patients (25.6%) experienced adverse drug reactions; the most frequently reported were increased hematocrit (52%) in 10 patients (7.5%), injection-site hemorrhage in 6 patients (4.5%), injection site bruising in 4 patients (3.0%), and increased prostate-specific antigen in 4 patients (3.0%). By week 26, mean systolic and diastolic blood pressure (BP) measured in the clinic increased by 3.4 mmHg (125.6-129.0 mmHg) and 1.8 mmHg (78.2-80.0 mmHg), respectively, from baseline. At week 12, ABPM showed 24-hour mean systolic and diastolic BP increases of 3.7 mmHg and 1.3 mmHg, respectively. All measured lipid fractions were below baseline levels at week 26. T, TE, dihydrotestosterone, and estradiol increased from weeks 1-12. T trough levels ranged from 300-650 ng/dL (10.4-22.5 nmol/L) in 82.4% and 83.2% of patients at weeks 12 and 26, respectively. Of the 965 assessed injections, mild pain was reported by 1 patient.
Clinical Implications: Dosing with SCTE is well-tolerated overall, yet associated with a numerically small mean systolic BP increase
Strengths & Implications: This study used a standardized ABPM protocol, confirming a numerically small systolic BP increase may be associated with reintroducing therapeutic T exposure in hypogonadal men. It is unknown at this time whether this applies with all routes of T supplementation.
Conclusion: SCTE-AI has a favorable safety profile and is well-tolerated, with a stable PK profile.
Figure 1. Hourly mean changes from baseline in panel A SBP and panel B DBP in the safety population and panel C is the 24-hour mean change in SBP and DBP from baseline at 6 and 12 weeks. Treatment with SCTE-AI resulted in nonsignificant changes in both SBP and DBP. DBP ¼ diastolic blood pressure; SBP ¼ systolic blood pressure; SCTE-AI ¼ subcutaneous testosterone enanthate auto-injector.
Figure 2. Clinical laboratory values for the safety population: panel A shows the PSA (mg/L); panel B shows the hematocrit (%); panel C shows the total cholesterol (mg/dL); panel D shows the HDL-C (mg/dL); panel E shows the LDL-C (mg/dL); and panel F shows the triglycerides (mg/dL). Values are expressed as mean (SD). HDL-C ¼ high-density lipoprotein cholesterol; LDL-C ¼ low-density lipoprotein cholesterol; PSA ¼ prostate-specific antigen.
Figure 3. Panel A is the weekly pharmacokinetic profile of TT concentrations at weeks 1, 6, and 12 for patients in the PK substudy. Panel B is the weekly pharmacokinetic profile of TE concentrations at weeks 1, 6, and 12 for patients in the PK substudy. Panel C is the weekly PK profile of DHT concentrations at weeks 1, 6, and 12 for patients in the PK substudy. Panel D is the weekly PK profile of E2 concentrations at weeks 1, 6, and 12 for patients in the PK substudy. All groups represent the PK substudy population.
CONCLUSIONS
Overall, when considering clinical laboratory assessments, vital signs, clinical findings, and ADRs, this study demonstrates that once-weekly use of SCTE-AI in patients with TD is well tolerated and has a safety profile similar to other T products. Patients in the study were highly compliant and successfully self administered weekly injections with few remarkable injection-site reactions and reports of pain.
Attachments
Last edited:















