High Hematocrit Caused by Testosterone Replacement Therapy
By Nelson Vergel, B.S.Ch.E., M.B.A.
High hematocrit occurs when there is an excessive production of red blood cells. High hematocrit can cause the blood to become very viscous or "sticky," making it harder for the heart to pump. High blood pressure, strokes and heart attacks can occur.
The association between testosterone replacement therapy and high hematocrit has been reported for the past few years as this therapy has become more mainstream. In addition to increasing muscle and sex drive, testosterone can increase the body's production of red blood cells. This hematopoietic (blood-building) effect could be a good thing for those with mild anemia.
Although all testosterone replacement products can increase the number of red blood cells, the study showed a higher incidence of higher hematocrit in those using intramuscular testosterone than topical administration (testosterone patch was the main option used -- no gels). Smoking has also been associated with polycythemia and may contribute to the effects of other risk factors.
Below is an excerpt from my book, Testosterone: A Man's Guide, further detailing the prevention and management of polycythemia.
Preventing and Managing Polycythemia
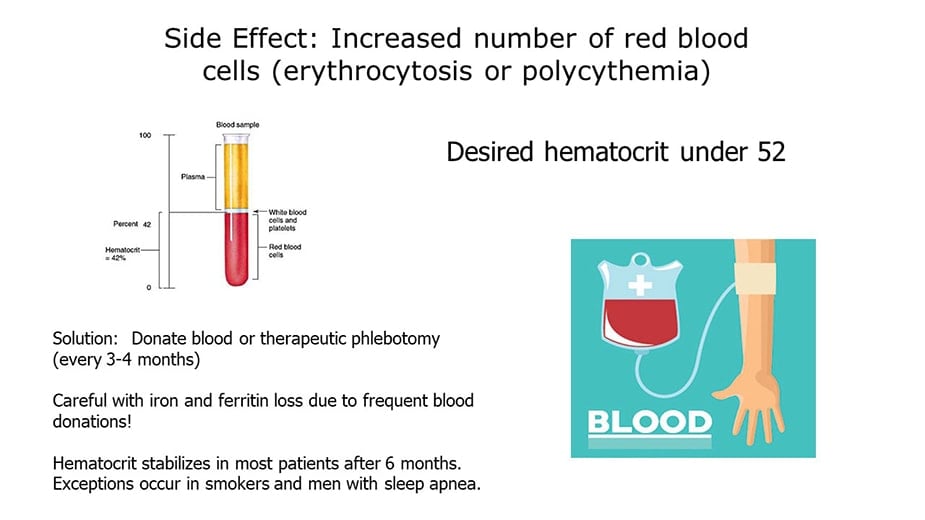
It's important to check patients' hemoglobin and hematocrit blood levels while on testosterone replacement therapy. As we all know, hemoglobin is the substance that makes blood red and helps transport oxygen in the blood. Hematocrit reflects the proportion of red cells to total blood volume. A hematocrit of over 52 percent should be evaluated. Decreasing testosterone dose or stopping it are options that may not be the best for assuring patients' best quality of life, however. Switching from injectable to transdermal testosterone may decrease hematocrit, but in many cases not to the degree needed.
The following table shows the different guideline groups that recommend monitoring for testosterone replacement therapy. They all agree about measuring hematocrit at month 3, and then annually, with some also recommending measurements at month 6 after starting testosterone (it is good to remember that there is a ban on *** blood donors in the United States).
Many patients on testosterone replacement who experience polycythemia do not want to stop the therapy due to fears of re-experiencing the depression, fatigue and low sex-drive they had before starting treatment. For those patients, therapeutic phlebotomy may be the answer. Therapeutic phlebotomy is very similar to what happens when donating blood, but this procedure is prescribed by physicians as a way to bring down blood hematocrit and viscosity.
A phlebotomy of one pint of blood will generally lower hematocrit by about 3 percent. I have seen phlebotomy given weekly for several weeks bring hematocrit from 56 percent to a healthy 46 percent. I know physicians who prescribe phlebotomy once every 8-12 weeks because of an unusual response to testosterone replacement therapy. This simple procedure is done in a hospital blood draw or a blood bank facility and can reduce hematocrit, hemoglobin, and blood iron easily and in less than one hour.
Unfortunately, therapeutic phlebotomy can be a difficult option to get reimbursed or covered by insurance companies. The reimbursement codes for therapeutic phlebotomy are CPT 39107, icd9 code 289.0.
Unless a local blood bank is willing to help, some physicians may need to write a letter of medical necessity for phlebotomy if requested by insurance companies. If the patient is healthy and without HIV, hepatitis B, C, or other infections, they could donate blood at a blood bank.
TRT Caused High Hematocrit: Where Can I Go for a Therapeutic Phlebotomy?


 www.redcross.org
www.redcross.org
 americasblood.org
americasblood.org
New York: Donation Locations

 www.redcrossblood.org
www.redcrossblood.org
For every unit of blood donated or extracted through therapeutic phlebotomy, there is a 2-3 point decrease in hematocrit. If your hematocrit is 56 and you want to bring him under 50, you would have to give 2 to 3 units of blood. However, taking this much blood out in one phlebotomy session may deplete ferritin and iron levels which can cause extreme fatigue. So, be conservative give 1 unit max even if you have to go more frequently. But be aware that blood donations done more frequently than every 2.5 months run the risk of lowering your ferritin and iron too much (which can cause fatigue), so make sure that you get a ferritin test to determine if you should take an iron supplement.
You can order a CBC test (includes hematocrit) and ferritin (after you donate blood) here: Blood tests
The frequency of the phlebotomy depends on individual factors, but most men can do one every two to three months to manage their hemoglobin this way. Sometimes red blood cell production normalizes without any specific reason. It is impossible to predict exactly who is more prone to developing polycythemia, but men who use higher doses, men with sleep apnea, and older men may have a higher incidence. It is important not to draw too much blood at once due to dramatic decreases in iron levels and ferritin that could cause fatigue.
Donating Blood: Red Cross List of Exclusions
Some doctors recommend the use of baby aspirin (81 mg) a day and 2,000 to 4,000 mg a day of omega-3 fatty acids (fish oil capsules) to help lower blood viscosity and prevent heart attacks. These can be an important part of most people's health regimen but they are not alternatives for therapeutic phlebotomy if the patient has polycythemia and does not want to stop testosterone therapy. It is concerning that many people assume that they are completely free of stroke/heart attack risks by taking aspirin and omega-3 supplements when they have a high hematocrit.
Although some people may have more headaches induced by high blood pressure or get extremely red when they exercise, most do not feel any different when they have polycythemia. This does not make it any less dangerous.

 www.excelmale.com
www.excelmale.com
WARNING:
Donating more than 1 unit every 2-3 months may lower your iron and ferritin levels and cause fatigue. That is why it is good to start donating when you are in the 51-52 hematocrit range. Also, the Red Cross and other organizations will reject donors with high hematocrit over 52. Those who get rejected can ask for a written order from their doctors and may have to pay to get phlebotomies.
Increasing testosterone enanthate versus hemoglobin, PSA and cholesterol:

Reference
More information:
Testosterone-Induced High Red Blood Cell Volume (Hematocrit) | Discounted Labs
balancing low ferritin levels and frequent donation.
Warning about frequent blood donations used to decrease hematocrit.
Donating Blood Prevents Heart Disease
Previous discussions on ways to manage hematocrit: grapefruit hematocrit excelmale.com site:www.excelmale.com - Google Search


By Nelson Vergel, B.S.Ch.E., M.B.A.
High hematocrit occurs when there is an excessive production of red blood cells. High hematocrit can cause the blood to become very viscous or "sticky," making it harder for the heart to pump. High blood pressure, strokes and heart attacks can occur.
The association between testosterone replacement therapy and high hematocrit has been reported for the past few years as this therapy has become more mainstream. In addition to increasing muscle and sex drive, testosterone can increase the body's production of red blood cells. This hematopoietic (blood-building) effect could be a good thing for those with mild anemia.
Although all testosterone replacement products can increase the number of red blood cells, the study showed a higher incidence of higher hematocrit in those using intramuscular testosterone than topical administration (testosterone patch was the main option used -- no gels). Smoking has also been associated with polycythemia and may contribute to the effects of other risk factors.
Below is an excerpt from my book, Testosterone: A Man's Guide, further detailing the prevention and management of polycythemia.
Preventing and Managing Polycythemia
It's important to check patients' hemoglobin and hematocrit blood levels while on testosterone replacement therapy. As we all know, hemoglobin is the substance that makes blood red and helps transport oxygen in the blood. Hematocrit reflects the proportion of red cells to total blood volume. A hematocrit of over 52 percent should be evaluated. Decreasing testosterone dose or stopping it are options that may not be the best for assuring patients' best quality of life, however. Switching from injectable to transdermal testosterone may decrease hematocrit, but in many cases not to the degree needed.
The following table shows the different guideline groups that recommend monitoring for testosterone replacement therapy. They all agree about measuring hematocrit at month 3, and then annually, with some also recommending measurements at month 6 after starting testosterone (it is good to remember that there is a ban on *** blood donors in the United States).
Many patients on testosterone replacement who experience polycythemia do not want to stop the therapy due to fears of re-experiencing the depression, fatigue and low sex-drive they had before starting treatment. For those patients, therapeutic phlebotomy may be the answer. Therapeutic phlebotomy is very similar to what happens when donating blood, but this procedure is prescribed by physicians as a way to bring down blood hematocrit and viscosity.
A phlebotomy of one pint of blood will generally lower hematocrit by about 3 percent. I have seen phlebotomy given weekly for several weeks bring hematocrit from 56 percent to a healthy 46 percent. I know physicians who prescribe phlebotomy once every 8-12 weeks because of an unusual response to testosterone replacement therapy. This simple procedure is done in a hospital blood draw or a blood bank facility and can reduce hematocrit, hemoglobin, and blood iron easily and in less than one hour.
Unfortunately, therapeutic phlebotomy can be a difficult option to get reimbursed or covered by insurance companies. The reimbursement codes for therapeutic phlebotomy are CPT 39107, icd9 code 289.0.
Unless a local blood bank is willing to help, some physicians may need to write a letter of medical necessity for phlebotomy if requested by insurance companies. If the patient is healthy and without HIV, hepatitis B, C, or other infections, they could donate blood at a blood bank.
TRT Caused High Hematocrit: Where Can I Go for a Therapeutic Phlebotomy?
List of blood donation agencies in the United States

Find a Location - Blood Centers of America
Find a Location QUICK LINKS Advanced Therapies Products & Services Supply Chain Solutions Whole Blood & Red Blood Cells Other Blood Components Cells Tissues
bca.coop

Give Blood
Giving blood is a simple thing to do, but it can make a big difference in the lives of others. Make a blood donation appointment with the American Red Cross today.
Find a Blood Center
Easily find your nearest community blood center and schedule a blood donation appointment today.
New York: Donation Locations
- Alabama
- Alaska
- Blood Bank of Alaska[9]
- Arkansas
- California
- Colorado
- St. Mary's Hospital Blood Center[18]
- Delaware
- Blood Bank of Delmarva[19]
- Florida
- Innovative Transfusion Medicine[20]
- LifeSouth Community Blood Centers[21]
- OneBlood[22]
- SunCoast Blood Centers[23]
- Winter Haven Hospital Community Blood Center[24]
- Georgia
- Atlanta Blood Services[25]
- LifeSouth Community Blood Centers[8]
- Shepeard Community Blood Center[26]
- The Blood Connection[27]
- Hawaii
I-M
- Illinois
- ImpactLife (Formerly: Mississippi Valley Regional Blood Center)[29]
- Community Blood Services of Illinois[30]
- Rock River Valley Blood Center[31]
- Versiti Illinois[32]
- Indiana
- Iowa
- ImpactLife (Formerly: Mississippi Valley Regional Blood Center)[35]
- LifeServe Blood Center[36]
- Kentucky
- Louisiana
- Michigan
- Versiti Michigan[44]
- Mississippi
- Mississippi Blood Services[45]
- Missouri
- Community Blood Center (Kansas City)[46]
- Community Blood Center of the Ozarks[47]
- ImpactLife (Formerly: Mississippi Valley Regional Blood Center)[48]
N-R
- Nebraska
- Nebraska Community Blood Bank[49]
- New York
- ConnectLife
- New York Blood Center[50]
- North Carolina
- The Blood Connection[27]
- Ohio
- Oklahoma
- Oregon
- Bloodworks Northwest[56]
- Pennsylvania
- Rhode Island
- Rhode Island Blood Center[60]
S-Z
- South Carolina
- Tennessee
- Texas
- Utah
- Virginia
- Washington
- Cascade Regional Blood Services[77]
- Bloodworks Northwest[78]
- Wisconsin
- ImpactLife (Formerly: Mississippi Valley Regional Blood Center)[79]
- Blood Center of Northcentral Wisconsin[80]
- Memorial Blood Centers[81]
- The Community Blood Center[82]
- Versiti Wisconsin[83]

Eligibility Criteria Alphabetical Listing
Are you interested in donating blood? Find out if you are eligible to become a blood donor by viewing eligibility criteria, including issues such as medication, travel, pregnancy and more.
For every unit of blood donated or extracted through therapeutic phlebotomy, there is a 2-3 point decrease in hematocrit. If your hematocrit is 56 and you want to bring him under 50, you would have to give 2 to 3 units of blood. However, taking this much blood out in one phlebotomy session may deplete ferritin and iron levels which can cause extreme fatigue. So, be conservative give 1 unit max even if you have to go more frequently. But be aware that blood donations done more frequently than every 2.5 months run the risk of lowering your ferritin and iron too much (which can cause fatigue), so make sure that you get a ferritin test to determine if you should take an iron supplement.
You can order a CBC test (includes hematocrit) and ferritin (after you donate blood) here: Blood tests
The frequency of the phlebotomy depends on individual factors, but most men can do one every two to three months to manage their hemoglobin this way. Sometimes red blood cell production normalizes without any specific reason. It is impossible to predict exactly who is more prone to developing polycythemia, but men who use higher doses, men with sleep apnea, and older men may have a higher incidence. It is important not to draw too much blood at once due to dramatic decreases in iron levels and ferritin that could cause fatigue.
Donating Blood: Red Cross List of Exclusions
Some doctors recommend the use of baby aspirin (81 mg) a day and 2,000 to 4,000 mg a day of omega-3 fatty acids (fish oil capsules) to help lower blood viscosity and prevent heart attacks. These can be an important part of most people's health regimen but they are not alternatives for therapeutic phlebotomy if the patient has polycythemia and does not want to stop testosterone therapy. It is concerning that many people assume that they are completely free of stroke/heart attack risks by taking aspirin and omega-3 supplements when they have a high hematocrit.
Although some people may have more headaches induced by high blood pressure or get extremely red when they exercise, most do not feel any different when they have polycythemia. This does not make it any less dangerous.
Therapeutic phlebotomy due to TRT may be covered at a blood bank now - Excel Male TRT Forum
Going through some papers that I set aside to look at later and low and behold I found a letter from my local blood bank that was sent to me after I did a therapeutic phlebotomy (had to pay a small fee). Looks like the FDA has eased up on the use of blood donations that are needed due to TRT...
WARNING:
Donating more than 1 unit every 2-3 months may lower your iron and ferritin levels and cause fatigue. That is why it is good to start donating when you are in the 51-52 hematocrit range. Also, the Red Cross and other organizations will reject donors with high hematocrit over 52. Those who get rejected can ask for a written order from their doctors and may have to pay to get phlebotomies.

TRT Induced High Red Blood Cells: How to Manage Hematocrit
Since abnormally high hematocrit values can pose serious health problems, this article will analyze the correlation between testosterone therapy and high hematocrit. TRT, sleep apnea and smoking are c
Increasing testosterone enanthate versus hemoglobin, PSA and cholesterol:
Reference
More information:
Testosterone-Induced High Red Blood Cell Volume (Hematocrit) | Discounted Labs
balancing low ferritin levels and frequent donation.
Warning about frequent blood donations used to decrease hematocrit.
Donating Blood Prevents Heart Disease
Previous discussions on ways to manage hematocrit: grapefruit hematocrit excelmale.com site:www.excelmale.com - Google Search
Last edited by a moderator:




















