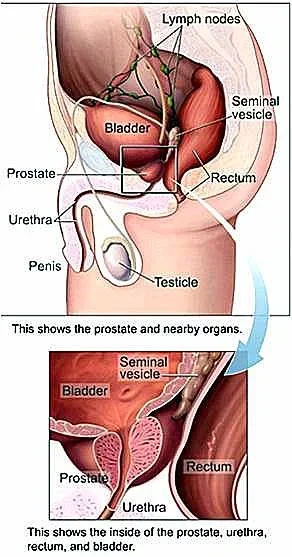
Men who have been treated for prostate cancer usually are hypogonadal and have all the symptoms of low testosterone. Most of these men are desperately looking for information on the safety of testosterone replacement since their doctors tell them they cannot use it. TRT is contraindicated for men with prostate cancer but few studies have looked at treating them after cancer treatment and remission. Many men who have been treated for prostate cancer with testosterone blocking agents and having their prostate removed lose bone density, stamina, muscle, libido, erectile rigidity, and have low mood. Fortunately, more studies and reviews are covering this issue that has been taboo until now.
These two paragraphs were taken from an excellent review on Medscape entitled "Testosterone in Hypogonadal Men Treated for Prostate Cancer: Testosterone Replacement in Men Treated for Prostate Cancer" by D. Landau; T. Tsakok; S. Aylwin and S. Hughes.
http://www.medscape.com/viewarticle/757420_4
"Testosterone replacement therapy after radical prostatectomy was the focus of Kaufman and Graydon's study in seven patients with hypogonadism over a maximum follow-up period of 12 years. They reported symptomatic improvement of hypogonadism, with no evidence of cancer recurrence. Agarwal and Oefelein administered TRT to a similar group of 10 patients and also reported that no PSA recurrences were associated with rises in mean serum testosterone from 197 to 591 ng/dl. In a study by Khera et al.,57 men received testosterone for an average of 36 months following radical prostatectomy – no increases in PSA were seen over a mean follow-up of 13 months. Of note is the larger study by Sathyamoorthy et al. in 133 postprostatectomy patients, 21 (16%) of whom had either Gleason score ≥ 8, positive margins or nodes. There were no significant PSA rises associated with increases in mean testosterone levels from 262 to 418 ng/dl over a 1 year follow-up. A prospective study of 22 patients by Nabulsi et al. [SUP][[/SUP]reported that one patient (4·5%) experienced biochemical recurrence after TRT postradical prostatectomy.
Despite the intuitive concern that residual prostate tissue is more likely to result in residual microscopic disease susceptible to androgen stimulation, some studies evaluating the use of TRT after radiotherapy and brachytherapy have also produced encouraging data. Sarosdy followed 31 men receiving testosterone after brachytherapy with a median 5 year follow-up, finding no incidence of cancer recurrence or progression. Morales et al. looked at five men post external beam radiotherapy and noted marked symptomatic improvement, with no biochemical recurrence for up to 27 months. Davila et al. analysed PSA response after TRT in a split cohort of 20 patients after either prostatectomy or external beam radiation therapy. There were no biochemical recurrences and no differences between the two groups in PSA levels after testosterone replacement. Finally, Leibowitz et al reported a retrospective study of a heterogeneously treated population of 96 patients. 60% of participants had not received definitive local therapy and consequently had both malignant and benign prostatic tissue in situ; notably, 11 men in the study group had metastatic disease in remission after ADT. After a mean treatment duration of 15 months, 41 men (43%) had PSA progression. This high rate of PSA progression can in part be explained by the relatively high proportion of patients with aggressive disease (25% had Gleason score ≥ 8). However, it is also possible that stimulation of residual benign prostatic tissue contributed to the raised PSA levels, which in most cases returned to baseline following cessation of testosterone replacement. Elevated baseline PSA before testosterone replacement was associated with a significantly increased risk of PSA progression, serving as a reminder that this therapy should be restricted to patients in overt biochemical remission.
We have summarized a small number of mostly retrospective case series evaluating the safety of initiating TRT in post prostatectomy patients with negative margins and undetectable PSA levels. Overall, 42 of 381 (11%, range 0–43%) experienced biochemical recurrence, whilst the majority benefited from symptomatic improvement in their hypogonadism without adverse effects on PSA or tumour progression. It is likely that research in this field suffers from publication bias as well as the modest quality of most studies. Nevertheless, the data presented here are largely at odds with the longstanding taboo regarding the use of androgens in men with prostate cancer and again raises the question: is it of clinical consequence if a normal serum testosterone level is achieved in a prostate cancer survivor by natural or pharmacological means? Until more studies have been published on this subject, we should take a cautious and conservative approach."

Last edited by a moderator:














